Written By Adeel Abbas
What is accuracy?
Table of Contents
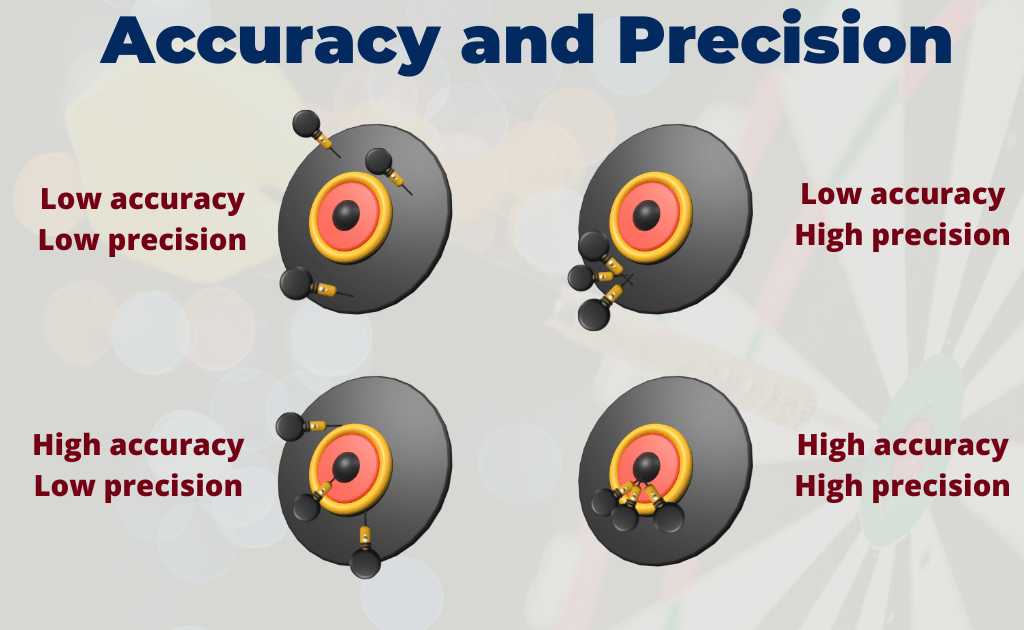
Accuracy refers to how close a measured value is to the true value.
Accuracy and precision are two fundamental concepts in analytical chemistry that are essential for obtaining reliable results.
What is precision?
Precision, on the other hand, refers to the reproducibility of a measurement. It is the degree of agreement among repeated measurements of the same quantity under the same conditions.
Precision is often represented as the standard deviation or coefficient of variation of a set of measurements.
Both accuracy and precision are important for ensuring that analytical data is reliable and can be used with confidence.
Also read: Difference between accuracy and precision
Examples of Accuracy and Precision
To better understand the concepts of accuracy and precision, let’s consider some examples. Suppose you want to measure the weight of a sample using a balance. If the balance is calibrated correctly and the weight of the sample is known, you can obtain accurate and precise measurements. If the balance is not calibrated correctly, or the weight of the sample is not known, the measurements may be inaccurate and imprecise.
Accuracy and precision are also important in other analytical techniques, such as chromatography and spectroscopy. In chromatography, the accuracy and precision of a measurement can be affected by factors such as the quality of the sample preparation and the chromatographic system’s performance.
In spectroscopy, accuracy and precision can be influenced by factors such as the quality of the instrument calibration and the choice of analytical method.
How to calculate accuracy and precision?
Accuracy refers to how close a measurement is to the true or accepted value. It is often represented as a percentage or error, which is the difference between the measured value and the true value.
To calculate accuracy and precision, follow these steps:
Calculate accuracy
- Subtract the measured value from the true value
- Divide the difference by the true value
- Multiply the result by 100 to get a percentage
- Accuracy = [(measured value – true value) / true value] x 100
Calculate precision
- Collect multiple measurements of the same quantity under the same conditions
- Calculate the mean or average of the measurements
- Calculate the standard deviation of the measurements
- Calculate the coefficient of variation by dividing the standard deviation by the mean and multiplying by 100
- Precision = (standard deviation / mean) x 100
By calculating accuracy and precision, you can evaluate the reliability of analytical data and identify any sources of error or variability.
Examples to calculate Accuracy and Precision
Let’s say we want to measure the weight of a small object using a digital scale. The true weight of the object is 10 grams.
We measure the weight of the object three times and get the following values: 9.8 grams, 10.2 grams, and 10.1 grams.
Subtract the measured value from the true value:
- 9.8 grams – 10 grams = -0.2 grams
- 10.2 grams – 10 grams = 0.2 grams
- 10.1 grams – 10 grams = 0.1 grams
Divide the difference by the true value:
- (-0.2 grams / 10 grams) x 100 = -2%
- (0.2 grams / 10 grams) x 100 = 2%
- (0.1 grams / 10 grams) x 100 = 1%
Multiply the result by 100 to get a percentage:
Accuracy values: -2%, 2%, 1%
We use the same three measurements from above: 9.8 grams, 10.2 grams, and 10.1 grams.
Calculate the mean or average of the measurements:
(9.8 + 10.2 + 10.1) / 3 = 10 grams
Calculate the standard deviation of the measurements:
Standard deviation = √[(9.8 – 10)^2 + (10.2 – 10)^2 + (10.1 – 10)^2] / (3 – 1)
Standard deviation = 0.200 grams
Calculate the coefficient of variation by dividing the standard deviation by the mean and multiplying by 100:
Coefficient of variation = (0.200 / 10) x 100 = 2%
Therefore, the accuracy of the measurements ranges from -2% to 2%, which indicates that the measurements are relatively close to the true value. The precision of the measurements is 2%, which indicates that the measurements are consistent and have a low amount of variability.
How to Improve Accuracy and Precision in Analytical Chemistry
To improve accuracy and precision in analytical chemistry, it is essential to understand the sources of errors and to take appropriate steps to minimize them. The following are some general tips for improving accuracy and precision in analytical chemistry:
- Ensure that the analytical instrument is properly calibrated and maintained
- Use appropriate sample preparation techniques to ensure that the sample is homogeneous and representative
- Use appropriate quality control measures, such as using standard reference materials and repeating measurements
- Follow standardized analytical procedures and protocols
- Ensure that the laboratory environment is properly controlled, with stable temperature and humidity conditions
Importance of accuracy and precision in chemistry
As an analytical chemistry weight balance expert, I understand the importance of accuracy and precision in chemistry. Accuracy and precision are critical components of scientific measurement, and they play a crucial role in ensuring the quality and reliability of chemical analysis.
- Accuracy refers to how close a measurement is to the true or accepted value. In analytical chemistry, accuracy is crucial in obtaining reliable data that can be used to draw valid conclusions. For instance, when measuring the concentration of a solution, accurate measurements are essential to ensure that the results are reliable and reproducible. Without accuracy, the results obtained may be inaccurate, leading to incorrect conclusions and potential harm to human health and the environment.
- Precision, on the other hand, refers to how close measurements are to each other. Precise measurements are essential in chemistry as they help to minimize random errors and ensure that the results obtained are consistent and reproducible. When analyzing a sample, the precision of the measurement is crucial in detecting small changes in the sample’s composition. For instance, when analyzing environmental samples for pollutants, precise measurements are essential to determine the concentration of the pollutant accurately.
- The importance of accuracy and precision in chemistry cannot be overstated. Inaccurate or imprecise measurements can lead to incorrect conclusions, wasted resources, and potential harm to human health and the environment. Therefore, it is critical to use accurate and precise measurement techniques when conducting chemical analysis.
- In addition to ensuring the reliability of the results, accuracy and precision also help to maintain the integrity of the scientific method. Scientific research depends on accurate and precise measurements to validate hypotheses and support scientific theories. Without accurate and precise measurements, scientific research cannot progress, and the validity of scientific theories can be called into question.
- In conclusion, accuracy and precision are fundamental concepts in chemistry, and they play a critical role in ensuring the quality and reliability of chemical analysis. As an analytical chemistry weight balance expert, I understand the importance of accuracy and precision in chemical analysis and the impact they have on scientific research. It is essential to use accurate and precise measurement techniques in chemistry to obtain reliable data, validate hypotheses, and support scientific theories.
Conclusion
Accuracy and precision are essential concepts in analytical chemistry, which are important for ensuring the reliability of analytical data. In this article, we have provided a comprehensive explanation of these concepts, including definitions, examples, and differences.