LEARNING OBJECTIVES
Table of Contents
In this article, the author has explained What is analytical chemistry, history and its applications in everyday life.
Analytical chemistry is the study of chemical analysis through analytical instruments. It can be used to identify, measure and quantify different substances in a sample.
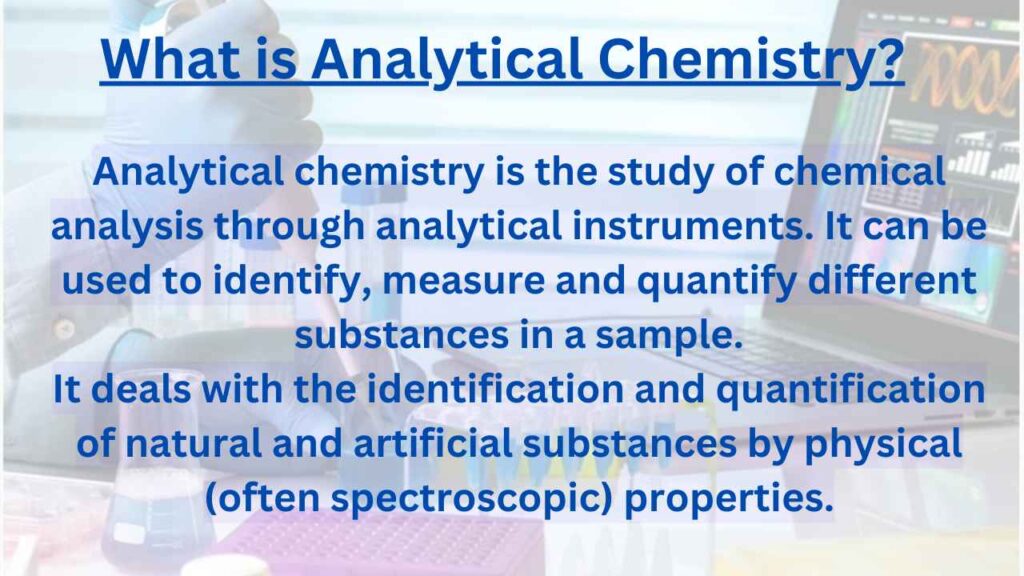
Analytical chemistry is a branch of chemical science that deals with the identification and quantification of natural and artificial substances by physical (often spectroscopic) properties.
Analytical chemists often specialize in one or more sub-disciplines such as analytical toxicology, food analysis, clinical pharmacology, environmental chemistry, polymer analysis, etc.
Analytical chemistry is the introduction of chemical methods to solve problems. In today’s world, analytical chemistry is a very important part of science as it solves so many problems for people and helps them in their daily life.
Analytical Chemistry has been split into two categories: qualitative analysis and quantitative analysis which are used widely by chemists.
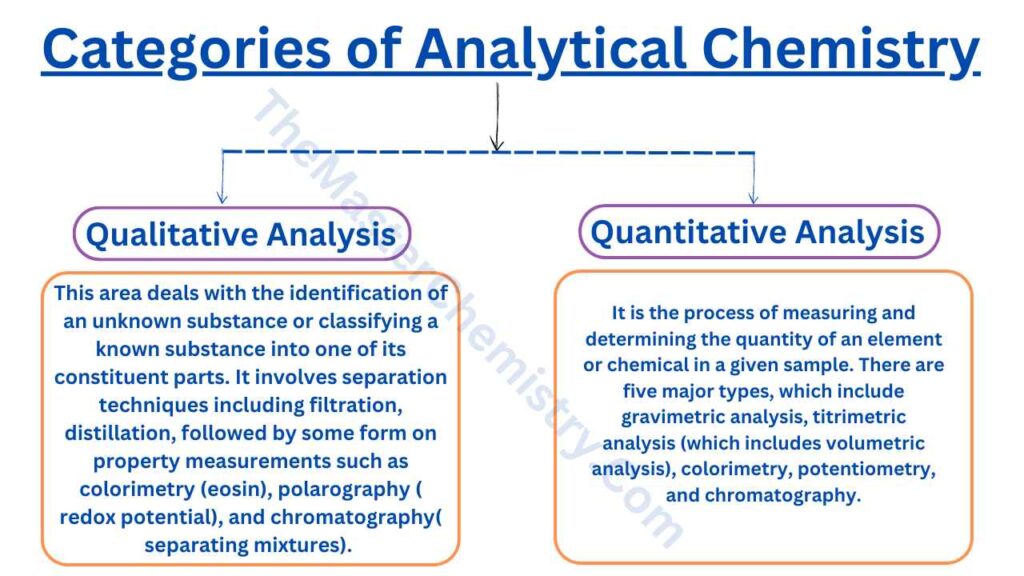
Qualitative Analysis
This area deals with the identification of an unknown substance or classifying a known substance into one of its constituent parts. It involves separation techniques including filtration, distillation, etc., followed by some form on property measurements such as colorimetry (eosin), polarography ( redox potential), and chromatography( separating mixtures). The main aim is to identify unknown compounds using properties like melting point, boiling point, and solubility.
Quantitative analysis
The process of measuring and determining the quantity of an element or chemical in a given sample. There are five major types, which include gravimetric analysis, titrimetric analysis (which includes volumetric analysis), colorimetry, potentiometry, and chromatography.
History of analytical chemistry
Analytical chemistry has a long history. In the past, chemists were often also physicists and had to learn how to measure physical quantities such as weight and volume. For example, Lavoisier discovered that water is not an element but rather a chemical compound of oxygen and hydrogen with the formula H20. He made this discovery by carefully measuring mass changes when reacting pure water into burning hydrogen gas (H) and de-combusting oxidized iron in the air (O).
However, analytical chemistry is more than just knowing what elements make up the matter; it now studies all types of substances regardless if they are metals or minerals, natural products like food or drugs, biomolecules like proteins or nucleic acids as well as complex mixtures like soil or waste water.
Scope of analytical chemistry
Analytical chemistry is the study of chemical processes involving a vast array of instruments and techniques. In practice, it can be broken up into several sub-disciplines.
Instrumental analysis involves using instrumentation to quantify matter through various chemical reactions or physical properties such as density or refractive index.
Instrumental analysis forms much of the basis for standardized measurements in experimental science and serves thousands of important roles today including testing drinking water quality and air pollutant levels at local environmental monitoring stations worldwide.
Modern instrumental methods are broadly grouped into two categories: analytical chemistry – where chemists develop highly specific procedures to directly detect, identify, and quantify individual types (elements) or groups (functional classes) of substances; and chemical process analysis – where chemists focus on studying chemical reactions, where the products are not necessarily one substance (element), but rather a mixture of substances.
Analytical chemistry is closely related to physical chemistry in that both fields involve the study and use of instruments to separate, identify or quantify matter.
While analytical chemists work primarily with samples from unknown sources using tools such as chromatography and spectrometry, physical chemists may employ these same techniques without any expectation of an immediate application – instead of focusing more broadly on developing new approaches for measuring phenomena like superconductivity or magnetism at their most fundamental levels.
In short, while it might be said that instrumental analysis combines elements from both pure and applied science within itself – so too does all modern experimental science depend heavily upon instrumentation to provide quantitative data.
A particular subfield of analytical chemistry is chemical analysis, which focuses on measuring properties of materials that are specific to a single substance (element). While the concept might seem simple enough, it can be quite complex in practice as many substances cannot simply be dissolved into solution and quantitatively analyzed due to their natural state or sample introduction techniques; instead of requiring some form of separations prior to introduction with instruments like chromatography
Another important field within instrumental analysis involves developing new methods for efficient sampling – often referred to as environmental forensics. Indeed, nearly all forms of modern industrialization depend heavily upon resource extraction, refinement, and processing using potentially hazardous chemicals; thus analytic chemists have been working closely with biologists since at least World War II to develop new ways of monitoring water quality, air pollution levels and human tissue for evidence of chemical exposure.
The final sub-field in the modern era involves developing methods that allow instruments or samples themselves to be miniaturized so they may be employed outside a laboratory setting with portable devices – sometimes referred to as “lab on a chip” technology. These techniques are often useful for medical applications such as testing blood glucose levels, but also have serious environmental implications
Applications of analytical chemistry in our daily life
Analytical chemistry is one of the most important fields in science. It has applications everywhere, from detecting pollutants to testing food for toxicity and vitamins.
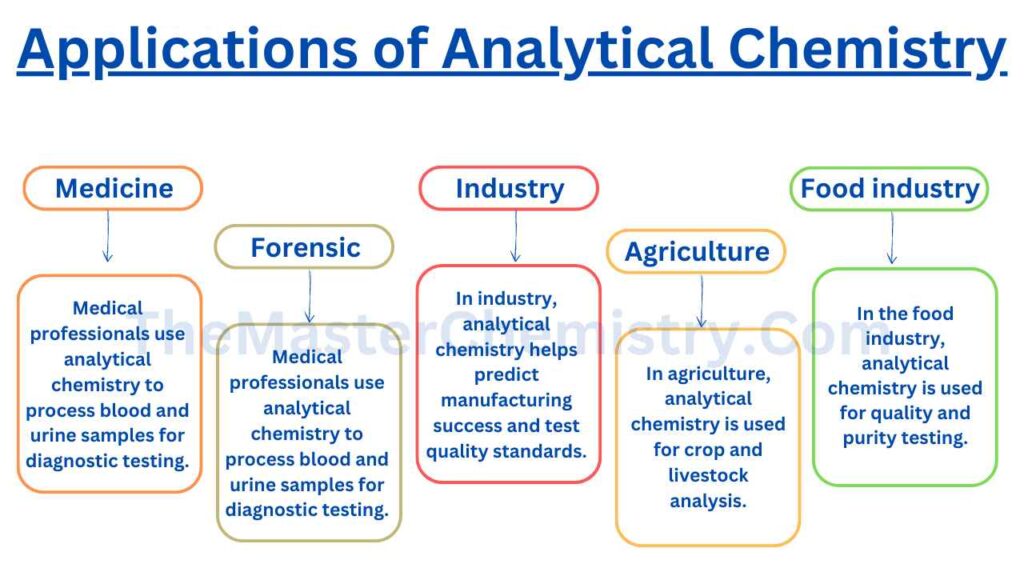
The first use of analytical chemistry is in the field of medicine.
It is used in hospitals and clinics to process blood samples for testing DNA, viruses, bacteria, etc., as well as perform urine analysis tests among many others. This allows doctors to come up with better medication or diagnoses for their patients depending on results that are achieved from using analytical chemistry techniques such as chromatography and spectroscopy.
Analytical chemistry also plays a huge role in forensics where it can be used to solve crimes by providing evidence about what happened at the scene of crime through chemical substances found there which would have otherwise remained unknown without this technique being applied successfully. DNA profiling has revolutionized forensic science significantly over recent years making use of these technologies extensively towards solving crimes worldwide where before it was almost impossible due to lack of any evidence.
uses of analytical chemistry in industry
This area is very useful because from being able to know how much of each material you have you can predict if your manufacturing process will be successful at producing the desired product within quality standards since quantities cannot always be seen by simply looking; sometimes they must be measured (e.g.: in a processing plant, you can’t see how much material is in the silos but there’s machinery that measures it).
uses of analytical chemistry in agriculture
Analytical chemistry is being used in different fields. In agriculture, analytical chemistry for crop analysis and livestock testing has been used in this area. In agriculture, analytical chemists can be used as crop analysts which means they have to analyze crops from soil fertility tests until mineral analysis in order to find out what kind of fertilizers need those crops so as not to lose their quality during seasons.
uses of analytical chemistry in the livestock industry
Analytical chemistry has a variety of applications in the livestock industry. It is used to test the quality of animal feed, identifying various species in livestock products, and also for testing contaminants
Application of analytical chemistry in food industry
Analytical chemistry is used extensively in the food industry for testing the quality and purity of various products. Some common techniques include chromatography, spectroscopy, mass spectrometry (MS), atomic absorption analysis (AAS), and infrared spectroscopy (IR).
One application could be measuring the fat content of a product by using high-performance liquid chromatography with evaporative light-scattering detection which separates out different substances based on their size and polarity.
The detector will then measure how much each substance returns scattered light at a given wavelength to produce an absorbance spectrum as shown below. From this scientists can determine if it contains any particular compound or not as well as its concentration via Beer’s Law.
In addition to this, there are various other techniques that can be used to further investigate the chemical components of food products. For example, gas chromatography (GC) is often used in combination with mass spectrometry (MS), which separates out different substances based on their boiling point and polarity.
The GC column then heats up each substance until it reaches its individual boiling point or ‘boiling range’ at which time they evaporate into a detector where molecules are ionized; positively charged ions indicate an even number of carbon atoms while negatively charged ions denote odd numbers of carbon atoms. From here scientists can determine what kind of compound has been released as well as its concentration via Beer’s Law.
Application of analytical chemistry in metrology
One of the main applications is that analytical chemistry is used in metrology. In this application, measurements are needed to be precise and accurate over a long period of time. This may include mass or volume measurements for medical devices, chemical sensors, oil refineries, etc.
Safety precautions while dealing with analytical chemistry
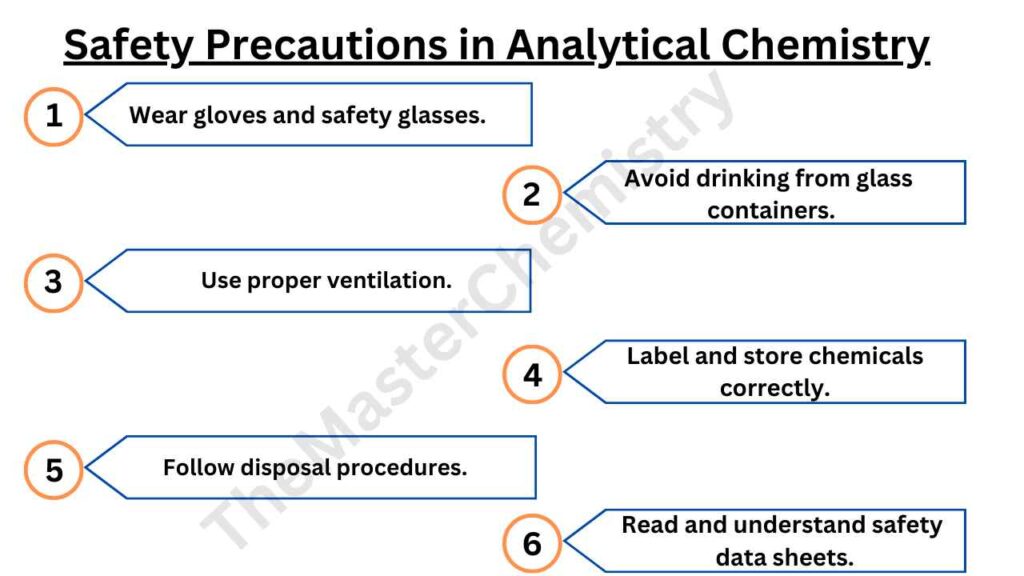
Working in a laboratory environment can be exciting, but it is also important to remember safety precautions while dealing with analytical chemistry. One of the most obvious safety precautions is to wear gloves.
This protects your hands from any chemicals that may harm you and ensures that you don’t contaminate samples by accidentally touching them with your bare skin. Another safety precaution is to never drink water or other liquids out of a glass container because there could be trace amounts of acid on the surface which can cause serious burns if consumed.
Another precaution to focus on in analytical chemistry laboratory safety is to protect your eyes. It’s important that you wear safety glasses in the lab at all times because chemicals can splash into your eyes and damage them, but also be aware of what’s happening around you when working with large samples or flammable liquids like ethers.
Conclusion
As you can see, analytical chemistry is a broad field that has many uses and applications. We hope we’ve given you an idea of what analytical chemistry entails and how it might be useful to different industries.
If this blog post has left you wanting more information on the subject matter, please comment below and ask any questions! Our team of experts will answer them as soon as possible.
Read all articles related to Analytical chemistry
- Sources of Errors in Titration
- Limit of Detection ( LOD): Master it for Scientific analysis
- Accuracy vs Precision- Know The Differences between Both
- Accuracy and Precision- Definition, solved examples and application
- Redox Titration: Principle, Types, Indicators, Applications, and Advantages
- What is Yield?-Definition, Types, and Examples
- Iodometric Titration: Principle, Example, Advantages
- Differences between Actual Yield and Theoretical Yield
- Betaine HCl – An Overview
- Tips for Improving the Accuracy of Acid-Base Titration
- How to choose indicator for acid base titration
- Column Chromatography-Principle, Types, Applications
- Gas chromatography-intro, principle, working, applications
- Chemistry of Toothpaste
- Triple point in chemistry | Triple point of water and CO2
- Chromatography-Principle, types, uses and History
- Synthetic Indicators-Examples, working, uses and cons
- Natural indicators-Examples, working and uses
- Acid base titration-Working principle, Process, types and indicators
- What are precautions during titration process in lab?
- Uses of titration in industries
- Why is titration important in chemistry? Themasterchemistry
- How is distribution law modified by change in Molecular state?
- How to prepare potassium chromate indicator?
- Analytical Chemistry, introduction, history and applications
- Limiting reactant- Examples, Problems & method to find limiting reactant
- Stoichiometry and yield of balanced chemical reactions
- Distribution law in chemistry-Definition, explanation, and applications
- Determination of molecular and empirical formula by combustion analysis
- Experimental techniques in chemistry-Filtration, Crystallization, Sublimation, Solvent extraction, Chromatography