When we study organic chemistry, we see a reaction condition called reflux. Many students do not understand the meaning of reflux. Reflux is an important experimental technique in chemistry.
Many organic chemical reactions take a long time to complete, so heat is applied in order to speed them up. High vapor pressures and low boiling points are what organic compounds are known for.
They become flammable and cause explosions when heated to a certain extent. The application of heat needs to be done in a specific way to overcome the issue of drying the reaction vessel.
In chemistry, reflux involves heating a chemical reaction for a specific amount of time while cooling it using a condenser. The condensation of the vapors above the reaction returns to the flask. The temperature of the reaction remains constant.
What types of reactants can be used in reflux experiment?
Table of Contents
Both liquids and solid reactants can be used in reflux experiments. The temperature at which the reaction is heated is determined by the boiling points of the solvents and the reflux ring.
Apparatus for reflux in chemistry
A reflux apparatus allows for facile heating of a solution, but without the loss of solvent that would result from heating in an open vessel. In a reflux setup, solvent vapors are trapped by the condenser, and the concentration of reactants remains constant throughout the process.
See the image below to understand the reflux apparatus.
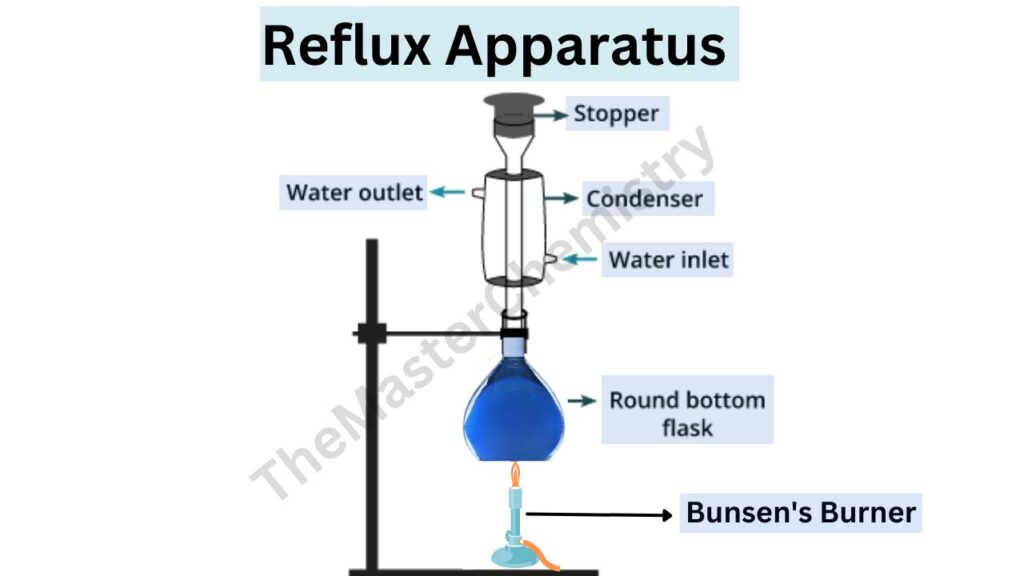
What is the purpose of reflux in chemistry?
The main purpose of refluxing a solution is to heat a solution in a controlled manner at a constant temperature. For example, imagine that you want to heat a solution to 60oC for one hour in order to conduct a chemical reaction.
It would be difficult to maintain a warm water bath at 60oC without special equipment, and it would require regular monitoring. However, if methanol was the solvent, the solution could be heated to reflux, and it would maintain its temperature without regular maintenance at the boiling point of methanol 65oC.
Why do we reflux in organic chemistry?
organic chemistry is a major branch of chemistry. In the research phase students in the chemistry lab works on natural products and synthetic chemistry. Both these research areas involve an excessive amount of solvents. So reflux is used to get the maximum solubility of the product in a solvent. It also saves the solvent that can be used again.
How to determine reflux temperature?
To determine the reflux temperature we need to attach the thermometer at the tip of the reflux condenser. There are the latest reflux condensers with an integrated thermometer at the top. Secondly, we can place the thermometer in the bathtub to measure the temperature of the solvent.
What is reflux ring?
A “reflux ring” is the upper limit where hot vapors are actively condensing or a space between two tubes of reflux Condenser where cool water is flow is known as reflux ring.
There are some solutions that you can use. With other solutions, the reflux ring is obvious with easily visible droplets. The reflux ring is subtle but can be seen with close observation, as liquid drips down the sides of the condenser, or as background objects appear distorted from the refraction of light through the liquid.
Steps of reflux process in chemistry
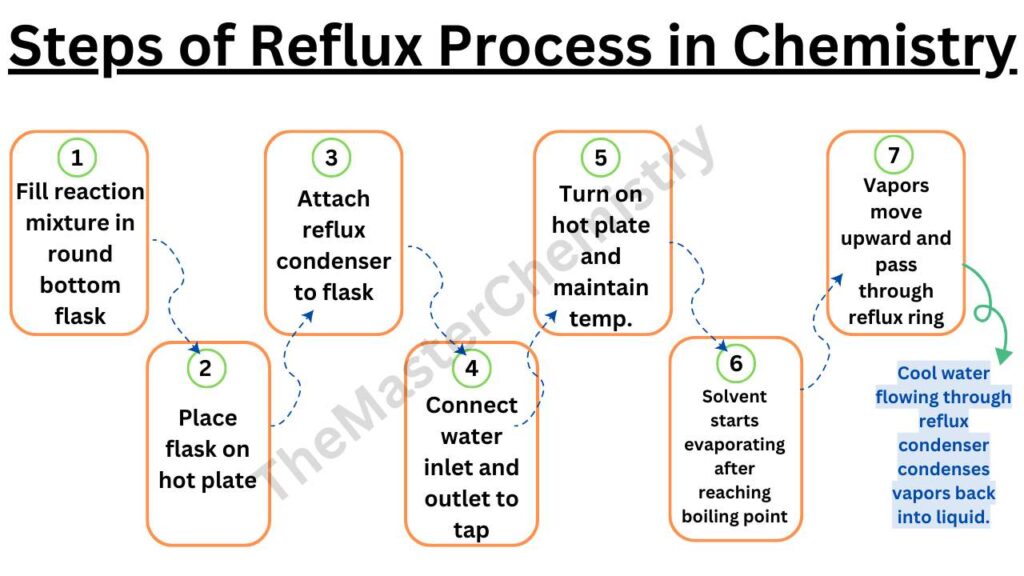
- First of all, a reaction mixture is filled in the round bottom flask.
- The flask is placed on the hot plate.
- A reflux condenser is attached to the flask containing the reaction mixture.
- The reflux condenser has a water inlet and an outlet attached to the tap or any water resources.
- The reaction is started by turning the hot plate on and the temperature is maintained according to the nature of the solvents in the flask.
- After the boiling point of the solvent is reached, it starts evaporating and vapors move in an upward direction.
- These vapors pass through the reflux ring (space between two tubes of reflux Condenser) where cool water is flowing.
- These solvent vapors are condensed back into liquid due to cold water in the reflux condenser.
This process is repeated continuously for hours.
Hope you understand what is reflux in chemistry. let us know in the comment section if you have any problems regarding this topic.