What are Electromagnetic Radiations?
Table of Contents
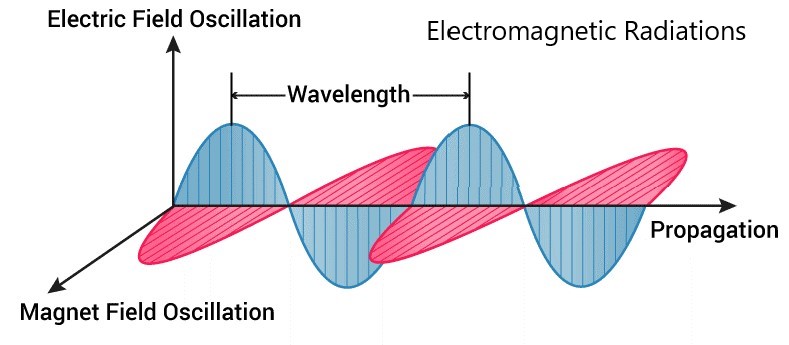
It is defined as a form of energy that is produced by the movement of charged particles through a matter or a vacuum. The magnetic and electric fields come at 90 to each other and the combined waves move to the other side of the field.
Discovery of Electromagnetic Radiations?
When electricity and magnetism were first thought of as separate forces. James Clerk Maxwell, the Scottish physicist, developed a combined theory known as electromagnetism in the year 1873. electromagnetic radiation and its properties will be discussed in this piece of article.
Properties of Electromagnetic Radiation
The electron radiations are released as particles called photons. These waves travel at the speed of light and are called bundles of light energy or quantized harmonic waves. There are different categories of energy based on the wavelength of the spectrum.
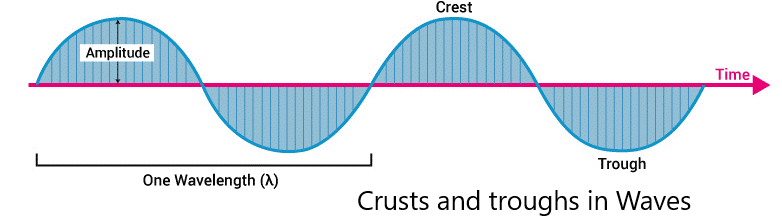
Some of the characteristics of magnetic and electric waves are wavelength, amplitude, and frequencies. The points mentioned below give some basic properties of Electromagnetic Radiation.
- They are able to travel through spaces that are empty. Waves other than microwaves have to travel through something.
- Solid, liquid, or gas will be needed for sound waves to travel through. The light’s speed of light is always constant.
- The symbol of wavelength is λ. It is the distance between troughs and crests. Types of electromagnetic radiations.
Types of electromagnetic radiations
1:Light
As a species, we have adapted our eyes to be sensitive to the most intense radiation emitted by the Sun, which is why the visible region of the spectrum is most familiar to us. The wavelength of the visible region can be found in the range of 400 to 700 nm.
Light is emitted when electrons in atoms change their state of motion. The state of electrons can have optical transitions. The color of the light tells us something. Information about the composition of light from the Sun and distant stars is given by the study.
2: Infrared
When atoms or Molecules change their motion, they emit infrared radiation, which has wavelength lengths longer than the visible (from 0.7 m to about 1 millimeter).
A change in the internal energy of an object that emits radiation can be observed as a change in the internal energy of the object that senses the radiation. In this case, heat radiation is an important means of heat transfer and it’s also sometimes called heat radiation.
3: Microwaves radiations
Microwaves can be considered short radio waves, with a typical wavelength in the range of 1 mm to 1 m. They are usually produced by the same people in electric circuits. As in the case of the microwave ovens. It is possible to show a microwave station that serves to relay telephone calls.
Microwaves can also come from extraterrestrial sources. As the universe expanded and cooled, the wavelength of the microwave background radiation was, which is believed to be the radiation associated with the “Big bang” fireball that marked the birth of the universe.
4: Radio Waves
The wavelength of a radio wave is 1 m. They are produced from electrons in wires.
The sensitivity of the detector and the distribution in space of the emitted radiation can be controlled by us. Traveling outward at the speed of light, the expanding wave-front of TV signals transmitted on Earth since about 1950 has reached approximately 400 stars, carrying information to their inhabitants, if any.
5. Ultraviolet
These are the radiations of wavelengths shorter than the visible begin with the ultraviolet, which can be produced in atomic transitions of the outer electrons, as well as in radiation from thermal sources such as the sun.
The main agent of this absorption is atmospheric ozone, which has been degraded in recent years as a result of chemical reactions with fluorocarbons released by aerosol sprays, refrigeration equipment, and other sources.
Short-term exposure to ultraviolet radiation causes sunburn, but long-term exposure can cause more serious effects, including skin cancer. Observatory carried into the earth by satellites are used for ultraviolet astronomy.
6: X -rays
When charged particles are decelerated, X-rays can be produced with a wavelength in individual transitions among the electrons of an atom, and they can also be produced.
X-rays from materials can be used to study their structure because they correspond to the spacing of the atoms. X-rays can penetrate soft tissue but are stopped by bones and other solid matter, which has led to widespread use in medical diagnosis.
7: Gama rays
The shortest wavelength (less than 10 pm) is the Gamma rays. Exposure to intense radiation can have a harmful effect on the human body, and they are the most penetrating of electromagnetic radiation.
These radiations can be emitted in transitions of an atomic nucleus from one state to another. Detection of such radiations in astronomy serves as evidence of particular nuclear processes.