In this article, the author has explained the following…
- Basics of mass spectrometry and detection of isotopes.
- The basic principle of the mass spectrometer
- Parts of mass spectrometer
- relationship between isotopes and atomic mass
What is Mass spectrometry?
Table of Contents
Mass spectrometry is an analytical method used to measure the exact masses of the different isotopes of an element.
Definition of mass spectrometry
Mass spectrometer
The instrument used for this purpose is called a mass spectrometer.
Definition of mass spectrometer
Initially, Aston’s mass spectrograph was used to identify the isotopes on the basis of their atomic masses.
Dempster’s mass spectrometer was designed for the elements found in the solid state.
The basic principle of mass spectrometer
In mass spectrometry a substance is first volatilized and then ionized with the help of high energy beam of electrons to form gaseous positive ions. These ions are separated on the basis of their mass to charge m/e ratio.
The result is recorded in the form of peaks which is in the form of a graph. On this graph m/e is plotted as abscissa) x-axis) and the relative number of ions as ordinate ( y-axis). This graph is called mass spectrum. It tells about the number, mass, and relative abundance of isotopes.
Parts of mass spectrometer
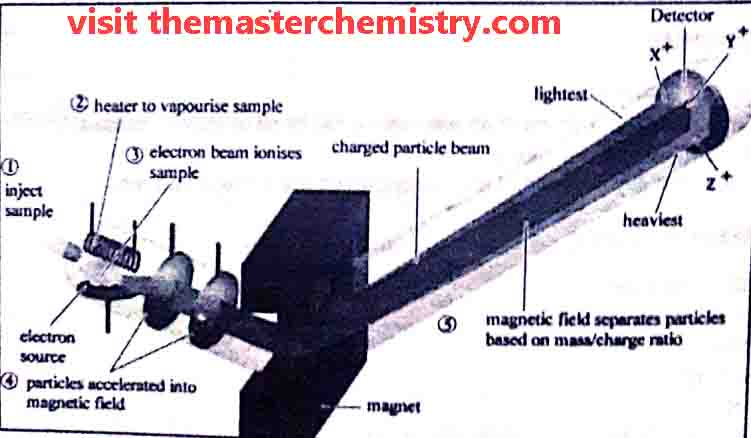
A typical mass spectrometer consists of following parts
- vaporization chamber
- ionization chamber
- Analyzer
- electrometer ( Ion collector)
- Detector, Amplifier, and recorder
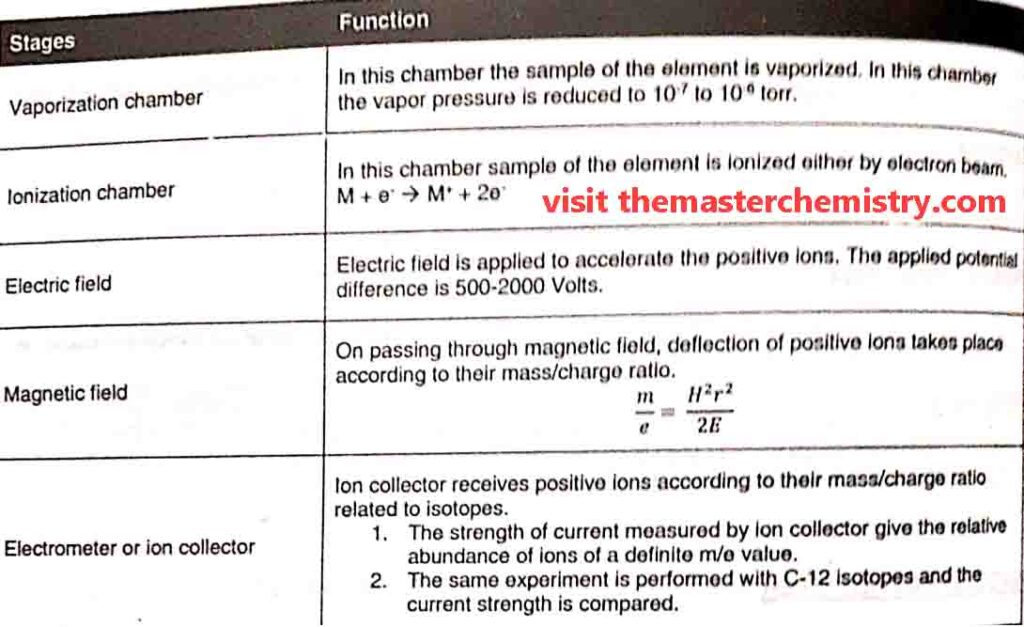
1: Vaporization Chamber
In the vaporization chamber the substance is first converted into vapour state. The pressure of these vapours is kept very low i.e. 10-6 to 10-7 torr.
2: Ionization chamber
In ionization chamber the fast moving electrons are bombarded upon the vapours. As a result gaseous atoms are ionized and positive ions are produced. These positive ions have different masses due to presence of different isotopes in them.
3: Analyzer
The analyzer separate ions on the basis of their m.e values in to steps.
i) Acceleration of ions
Each positive ion has its own m/e value. m/e is known as mass to charge value or ratio. A potential diference ( E ) of 500-2000 volts is applied between the perforated plates to accelerate the ions. Thus ions are strongly attracted towards the negative plate.
ii) Deflection of ions:
the ions are then passed through a strong magnetic field of strength “H”. It deflects the ions on the basis of their m/e value. Thus ions follow a circular path.
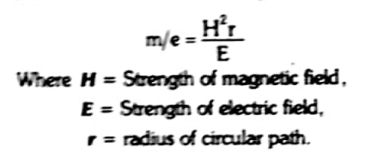
It represents that
If we Keep E constant, if H is increased, r also increases. Therefore, ions of a particular m/e charge value can be adjusted to fall at different place than before. The same results can also b e obtained by keeping H constant and change E.
Hence, we can sat that smaller the m/e value, smaller will be the r produced by magnetic field. Therefore, the ions having similar m/e value are separated into beams of ions.
4: Electrometer or ion collector
In mass spectrometerthe separated beams of ions are passed through slit one by one. Each beam contains ions of the specific m/e value which fall on electrometer or ion collector. The electrometer develops electric current. The strength of current for each beam gives the relative abundance of ions.
5: Detector, Amplifier, and recorder
If we talk about modern spectrometers the ions strike the detector to generate current which is then amplified and fed to the recorder. This recorder is connected to a computer which draws the peaks according to the intensity of m/e ratio.
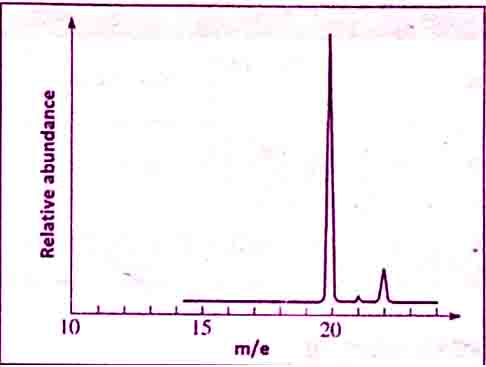
Mass spectrum or mass spectrograph
To understand this we will take the example of mass spectrum of Neon.
The number of isotopes: The three peaks in the mass spectrum show it contain three isotopes with relative isotopic masses, 20, 21, and 22 respectively.
The abundance of isotopes: The relative heights of the peaks give a information about the relative abundance of the isotopes.
Some other techniques for the separation of isotopes
- Gaseous diffusion
- Thermal diffusion
- Distillation
- Ultracentrifuge
- Electromagnetic separation
- Laser separation
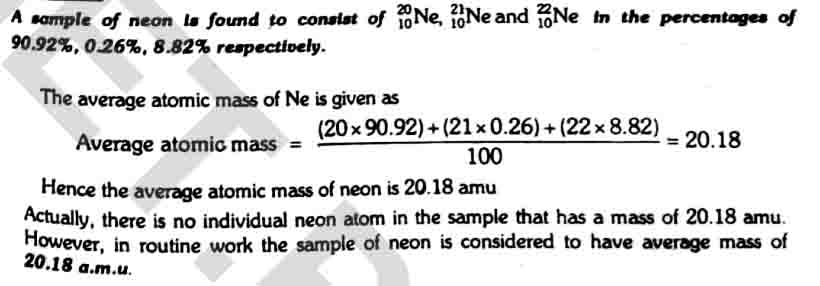
Relation between atomic mass and isotopes
The atomic masses of elements are represented as average atomic masses. These depend upon the number of isotopes of an element, their masses and their natural abundances. Therefore, most of the elements have fractional atomic masses.
Let others know about this