LEARNING OBJECTIVES
In this article, you will learn about the peculiar behavior of Lithium with explanation, properties, and nature of lithium, the difference between lithium and other alkali metals by professional author.
Lithium differs in many properties from other alkali metals. This difference from its group members is due to the following reasons.
a) The sizes of lithium atom and Li+ ion are very small as compared to the atoms and ions of other alkali metals.
b) Due to the very small size of Li+, it has a high charge density and high polarizing power. For this reason, it has a greater tendency to form covalent bonds.
c) Its ionization energy and electronegativity are the highest among its family members.
Difference of Lithium from other alkali metals
Table of Contents
There are certain factors where lithium differs from other group members as follows…
1: Hardness
Lithium is harder and lighter than other alkali metals.
2: Melting and boiling points
The melting and boiling points of lithium are higher than those of alkali metals.
3: Melting of lithium
Lithium can be melted in dry air without losing its brilliancy.
4: Covalent bonds
Li has the tendency to form covalent bonds with electronegative atoms, while other atoms of this group make the ionic bonds.
5: Solubility of salts
Some of the salts of lithium such as LiOH, LiF, Li2CO3, and Li3PO4 are insoluble in water. On the other hand, the corresponding salts of other alkali metals are soluble in water.
6: Reactivity
Li is the least reactive metal among all the alkali metals due to its small atomic size.
7:Burning in air
When Li is burnt in the presence of air, it forms only normal oxides such as Li2O. The other family members of group I-A form peroxides and superoxides.
8: Formation of oxides
The normal oxides of lithium (Li2O) dissolves in water quietly, while the oxides of other alkali metals dissolve in water more energetically.
9: Reaction with water
Li reacts with water in a slow rate, while other alkali metals react with water violently.
10: Basic strength of hydroxides
LiOH is less soluble in water and is a weak base, while the hydroxides of other alkali metals like NaOH and KOH are highly soluble in water and are strong bases.
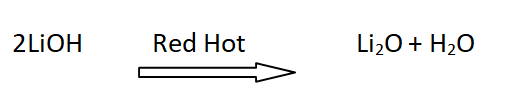
11: Stability of hydroxides of lithium
LiOH is decomposed at red hot forming Li2O . The hydroxides of other alkali metals sublime upon heating.
12: Stabilites of hydrides
LiH is more stable than the hydrides of other alkali metals.
13: Soluibilites of cholirides
LiCl is deliquescent and soluble in alcohol and pyridine. The chlorides of other alkali metals do not do so.
14: Heats of solutions of chlorides
LiCl has exothermic heat of solution, while the chlorides of Na and K have endothermic heat of solutions.
15: Hydrolysis of chlorides
LiCl can undergo hydrolysis but the chlorides of other alkali metals do not exhibit this property.
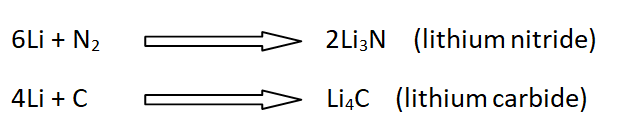
16: Reaction with N2, C, and Si
Li reacts with nitrogen, carbon, and silicon to give nitrides, carbides, and silicide.
For example,
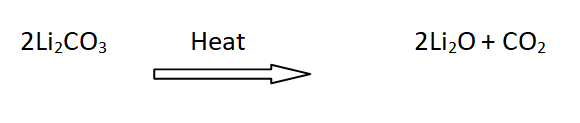
17: Decomposition of lithium carbonates
Li2CO3 is decomposed on heating to form Li2O and CO2, while carbonates of other alkali metals are stable towards heat.
For example
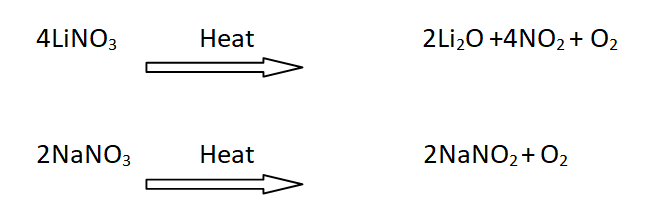
18: Decomposition of nitrates
When LiNO3 is heated it gives NO2 gas, while the nitrates of other alkali metals simply give oxygen.
For example
19: Reaction with acetylene
When acetylene is passed over strongly heated Li, it does not produce lithium acetylide. Other alkali metals form the corresponding metallic acetylides.
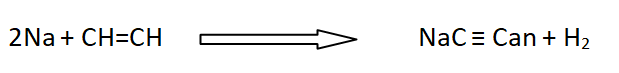
20: Complex formation
Lithium forms stable complex compounds. One of the stable complexes formed by lithium is Li(NH3)4.
Comparison of lithium with other alkali metals
