LEARNING OBJECTIVES
In this article, you will learn about the intermolecular forces such as dipole-dipole forces, dipole-induced dipole forces instantaneous dipole-induced dipole forces, or London dispersion forces with examples by a professional author.
Force in chemistry
Table of Contents
The attraction that exists within the molecules or between atoms of different molecules is known as force.
We know that atoms combine to form a molecule. There are two types of molecules depending on the type of forces.
Intermolecular forces and intramolecular forces.
Let us discuss about these in detail…
Intermolecular forces
The forces of attraction between separate molecules of substances are called intermolecular forces.
These forces are also known as Van der Waals forces.
- These forces are present among all types of atoms and molecules when they are close to each other.
- The physical properties of substances such as melting and boiling points depend upon the strength of intermolecular forces.
- These forces have no relation with valence electrons.
- These forces are weaker than intramolecular forces.
- For example Dipole-Dipole forces, London dispersion forces etc.
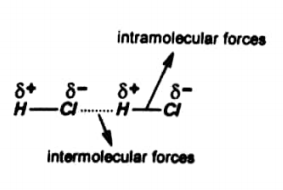
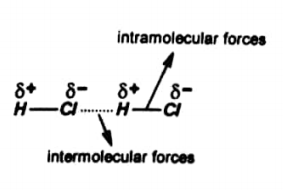
Intramolecular forces
The forces of attraction between atoms within a molecule are known as intramolecular forces.
For example:
- Chemical bonds i.e ionic bond, covalent bond, coordinate covalent bond.
- These have almost no relation to the physical properties of substances.
- These forces have a concern with valence electrons.
- These forces are stronger than intermolecular forces.
The question arises here why intramolecular forces are stonger than intermolecular forces?
Let us understand this with an example…
Consider HCl, in this a covalent bond is present between H and Cl. Both atoms complete their valence shell. Thus these tend to always remain together. Therefore, this linkage is very strong.
While in intermolecular forces only a weak electrostatic attraction is present between Cl– of one molecule and H+ of other molecule. So this linkage is weak.
Types of intermolecular forces
Intermolecular forces are also called van der Waals forces. These especially exist when the molecular are close to each other.
There are many types of intermolecular forces…..
- Dipole-Dipole forces
- Ion-dipole forces
- Dipole-induced dipole forces
- Instantaneous dipole-induced dipole forces of London forces
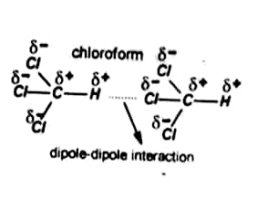
Dipole-Dipole forces
The electrostatic forces which are developed when a negative end of one polar molecule attracts the positive end of other molecules are called dipole-dipole forces.
To better understand this consider a bond between two different atoms such as H-Cl. In HCl the Cl is more electronegative than H. It attracts the shared electrons more towards itself. Thus Cl gets partial negative charge &– and H gets partial positive charge &+.
This type of bond is called a polar bond and the molecule is called a dipole.
Therefore, when molecules are close to each other, they tend to line up and attract each other. However, the thermal energy of molecules does not permit perfect alignment.
These forces are approximately one percent as effective as a covalent bond.
What factors affect dipole-dipole forces?
The strength of dipole-dipole forces depends upon two factors
Difference in electronegativity of the bonded atoms
Greater the electronegativity difference more polar is the bond. Therefore, stronger the dipole-dipole forces and vice versa.
For example dipole –dipole forces in Chloroform.
Distance between molecules
Larger the distance between molecule, weaker the dipole-dipole forces and vice versa.
For example in gases, molecules are widely separated therefore, these forces are very weak.
In liquids molecules are close to each other therefore these forces are stronger.
Effect of Dipole-Dipole forces on physical properties
The physical properties of substances such as melting and boiling point depends upon the strength of dipole-dipole forces.
Generally, stronger the dipole-dipole forces greater the values of thermodynamic properties of a substance like melting point, boiling point, heat of vaporization and heat of sublimation etc.
Dipole-Induced Dipole forces or Debye forces
The electrostatic forces of attraction between the permanent dipole of one molecule and the induced dipole of another molecule are called dipole-Induced dipole forces.
For example
These are present in a mixture of polar and non-polar molecules. In this mixture positive end of polar molecule attracts mobile electrons of non-polar molecules.
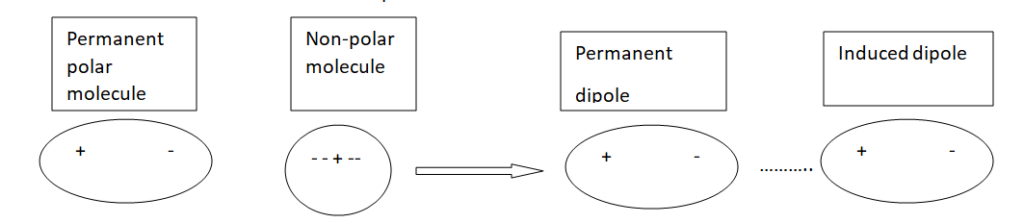
Thus dipole is induced in non-polar molecues. The forces of attraction between polar molecule and induced dipole is called dipole-induced dipole force of Debye force.
Instantaneous Dipole-induced Dipole forces or London Dispersion Forces
The momentary force of attraction between instantaneous dipole and induced dipole is called instantaneous dipole-induced dipole force or London dispersion force.
Non-Polar Helium He gas can be liquefied which shows that its molecules have forces of attraction. To account for such things, Fritz London a German physicist explained these forces in 1930. These are called London dispersion forces.
Example
These forces are more prominent in non-polar molecules such as H2, N2, and noble gases, etc. In polar molecules, attractive forces are due to the formation of the dipoles. However, in non-polar molecules dipoles are not present under normal conditions.
Mechanism of formation of London Dispersion Forces
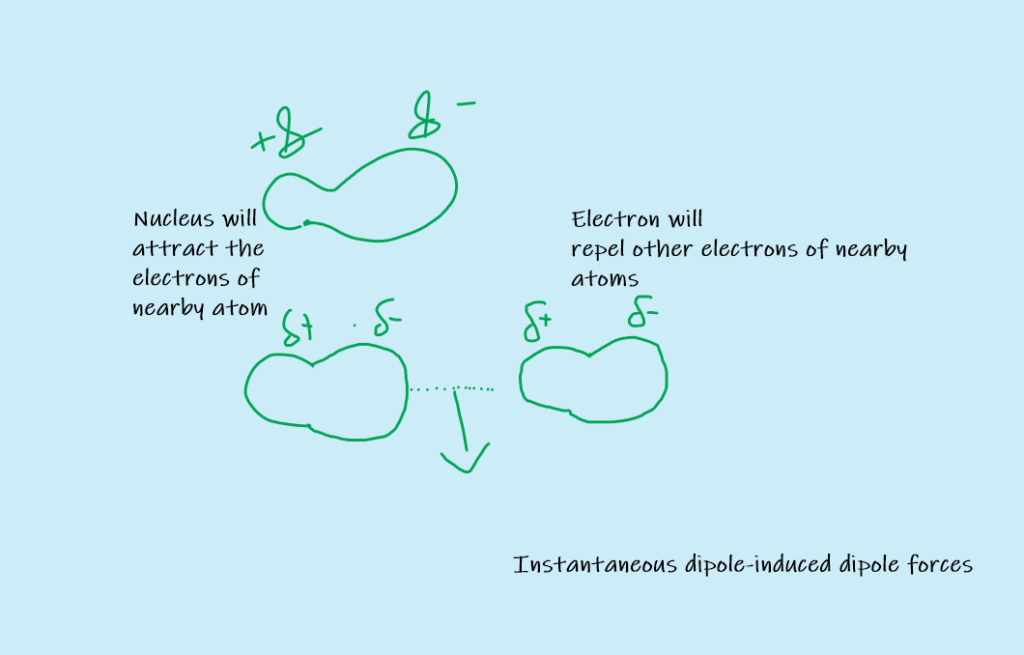
Consider non-polar Helium molecules. When two He molecules come closer to each other, their electrons repel each other and are pushed away. Therefore, a temporary dipole is produced in the molecules. Thus electron density of molecule is no more symmetrical and it becomes a dipole. This is called instantaneous dipole.
The positive end of the instantaneous dipole attracts electrons of another molecule. In this way, a dipole is induced in the nearby molecule as well. This is called induced dipole.
The force of attraction is then developed between instantaneous dipole and induced dipole. It is called instantaneous dipole-induced dipole force or London dispersion force.
The temporary dipole is finished very soon. However, a new dipole will appear in some other direction and thus weak forces are again developed between molecules.
These forces are present in all types of molecules (polar and non-polar). However, these are more prominent in non-polar molecules such as H2, Cl2, noble gases, etc.
London forces are weaker than dipole-dipole forces.
Polarization
The measurement of the extent to which the electron cloud can be distorted or polarized is called polarizability.
A specie ( atom, ion, and molecule) is said to be polarized if temporary dipoles are created in it by the distortion of electron cloud.
Which factors affect the London Forces?
Following factors affect the London forces…..
1: Size of Electronic cloud
Larger the size of atoms and molecules, dispersion is easy and polarizability is high. Hence London forces are stronger.
Examples:
Boiling points of Noble gases and halogens increases down the group in the periodic table
Noble gases are monoatomic gases and do not form covalent bonds among themselves because their outermost shells are complete.
In noble gases, electronic cloud size increase down the group in periodic table due to increase in atomic size. Thus, atoms are easily polarized down the group and develop strong London forces.
That’s why boiling points of noble gases increase down the group. Similarly boiling points of halogens also increases down the group.
Halogens have different physical states at room temperature.
Halogens are non-polar diatomic molecules. Fluorine, chlorine, and bromine are liquid while Iodine is solid.
It is because electronic cloud size increases down the group in periodic table due to increase in atomic size.
Thus atoms are easily polarized down the group, and develop strong London forces. Hence physical sates of halogens changes down the group and boiling points also increases. For example boiling point of Fluorine is 188.1 0C while of iodine is much greater than fluorine due to larger size.
2: Number of atoms in a molecule
Generally greater the number of atoms, stronger the London forces and vice versa.
Greater number of atoms means greater polarizability and hence stronger London forces.
For example
Ethan has a lower boiling point -88.6 0C than hexane 68.7 0C.
However, both ethane and hexane are non-polar. This is due to larger number of atoms in hexane which develops stronger forces of attraction than ethane.
Because larger hexane molecule has more places for attraction and greater polarizability than smaller ethane molecule. So it has stronger forces and higher boiling point. Further due to stronger forces, hexane is a liquid while ethane is a gas at room temperature.
The physical state of hydrocarbons changes with an increased molecular mass
The physical states of hydrocarbons change from gas to liquid to solid with increasing molecular mass. It is because larger molecules have more places for attraction and greater polarizability than smaller molecules. Therefore, these develop stronger forces. Hence melting and boiling points also increases with an increase in molecular masses.