Written By Adeel Abbas
Benzene will only add 2, 4, or 6 functional groups during an audition process, with no byproducts produced. The carbons at either end of a carbon-carbon double bond are often added to these additional processes, adding two portions of a simple molecule.
The stability of benzene and its compounds is substantially higher than anticipated. Because of its increased stability, benzene is less likely to undergo additional reactions. Electrophiles can target the more slackly held electrons. Therefore, the electrophilic substitution reaction is the typical reaction of benzene.
Some additional reactions of benzene derivatives are given below.
Chlorination of benzene
Table of Contents
Although substitution is more common, aromatic compounds may undergo addition if forcing conditions are used. When benzene is treated with an excess of chlorine under heat and pressure six chlorine atoms add to form benzene hexachloride (BHC). This process is also known as Halogenation of benzene.
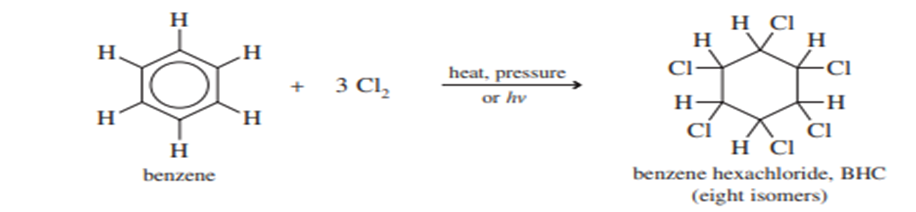
The addition of chlorine to benzene, believed to involve a free-radical mechanism, is normally impossible to stop at an intermediate stage. The first addition destroys the ring’s aromaticity, and the next 2 moles of Cl2 add very rapidly. All eight possible stereoisomers are produced in various amounts. The most important isomer for commercial purposes is the insecticide lindane, which is used in shampoo to kill head lice.
Catalytic Hydrogenation of Aromatic Rings
Catalytic hydrogenation of benzene to cyclohexane takes place at elevated temperatures and pressure. Often catalyzed by ruthenium or rhodium. Substituted benzenes react to give substituted cyclohexane; disubstituted benzenes usually give mixtures of cis and trans isomers.
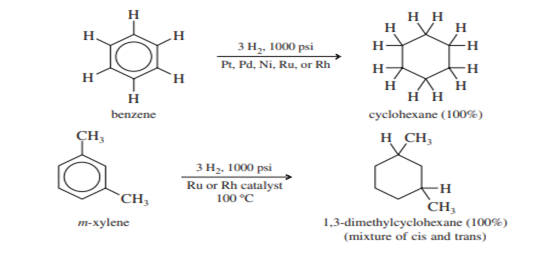
Catalytic hydrogenation of benzene is the commercial method for producing cyclohexane and substituted cyclohexane derivatives. The reduction cannot be stopped at an intermediate stage (cyclohexene or cyclohexadiene) because these alkenes are reduced faster than benzene.
Birch Reduction
In 1944, the Australian chemist A. J. Birch found that benzene derivatives are reduced to nonconjugated cyclohexane-1,4-dienes by treatment with sodium or lithium in a mixture of liquid ammonia and alcohol. The Birch reduction provides a convenient method for making a wide variety of interesting and useful cyclic dienes.
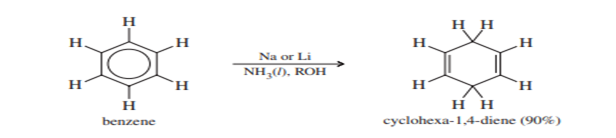
The mechanism of the Birch reduction is similar to the sodium/liquid ammonia reduction of alkynes to trans-alkenes. A solution of sodium in liquid ammonia contains solvated electrons that can add to benzene, forming a radical anion. The strongly basic radical anion abstracts a proton from the alcohol in the solvent, giving a cyclohexadienyl radical. The radical quickly adds another solvated electron to form a cyclohexadienyl anion. Protonation of this anion gives the reduced product.
Mechanism for birch reduction
The Birch reduction involves twice adding a solvated electron, followed by a proton, to the aromatic ring.
Preceding step: Formation of solvated electrons in the ammonia solution.
NH3 + Na ⇌ NH3 .e– (deep blue solution) + Na+
Steps 1 and 2: The addition of an electron, followed by a proton, forms a radical.
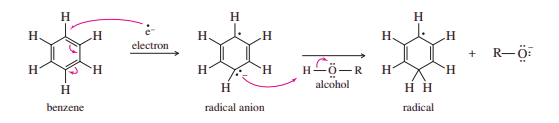
Steps 3 and 4: The addition of a second electron, followed by a proton, gives the product.
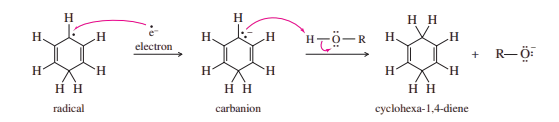
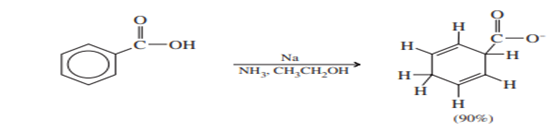
The two carbon atoms that are reduced go through anionic intermediates. Electron-withdrawing substituents stabilize the carbanions, while electron-donating substituents destabilize them. Therefore, reduction takes place on carbon atoms bearing electron-withdrawing substituents (such as those containing carbonyl groups) and not on carbon atoms bearing electron-releasing substituents (such as alkyl and alkoxyl groups).
A carbon-bearing electron-withdrawing carbonyl group is reduced.
A carbon-bearing electron-releasing alkoxyl group is not reduced.
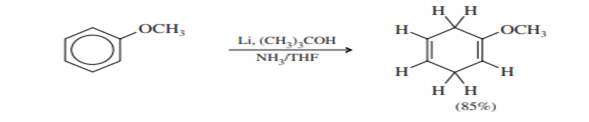
Substituents that are strongly electron-releasing (for example) deactivate the aromatic ring toward Birch reduction. Lithium is often used with these deactivated systems, together with a co-solvent (often THF) and a weaker proton source (tert-butyl alcohol). The stronger reducing agent, combined with a weaker proton source, enhances the reduction.
FAQs
What is the most common reaction of benzene and its derivatives?
Benzene and its derivatives are much more stable than expected. The extra stability means that benzene will less readily undergo addition reactions. The more loosely held electrons are open to attack by electrophiles. Hence, the characteristic reaction of benzene is an electrophilic substitution reaction.
Which benzene pathway is more favorable addition or substitution?
The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occurs in preference to addition reactions.
Is chlorination of benzene an addition reaction?
Chlorine adds to benzene in the presence of ultraviolet light. With methylbenzene under those conditions, you get substitution in the methyl group. That is energetically easier because it doesn’t involve breaking the delocalized electron system.
Is hydrogenation of benzene an addition reaction?
Hydrogenation is an addition reaction in which hydrogen atoms are added all the way around the benzene ring. A cycloalkane is formed.
What are the three types of addition reactions?
The different types of addition reactions are:
Nucleophilic addition reaction.
Electrophilic addition reaction.
Free radical addition reaction.
What is the two common addition reaction with benzene?
Benzene can participate in additional reactions with hydrogen and halogens (like chlorine and bromine), in hydrogenations and halogenations.