Written by Adeel Abbas
The introduction of a halogen into benzene is called the halogenation of benzene.
Definition of halogenation of benzene
Halogenation is an example of electrophilic aromatic substitution. In electrophilic aromatic substitutions, benzene is attacked by an electrophile which results in the substitution of hydrogen. Halogens are not sufficiently electrophilic to react with benzene.
Activation of Halogen
Table of Contents
A strong Lewis acid such as FeBr3 catalyzes the reaction. However by forming a complex with Br2 that reacts much like Br+. Bromine donates a pair of electrons to FeBr3, forming a stronger electrophile with a weakened Br—Br bond and a partial positive charge on one of the bromine atoms. Attack by benzene forms the sigma complex, giving the aromatic product and HBr, and regenerating the catalyst.
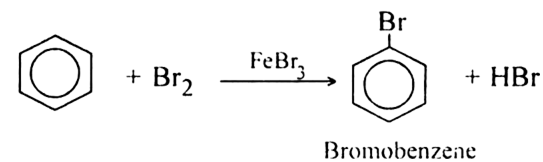
Mechanism
Step 1: Formation of a stronger electrophile.
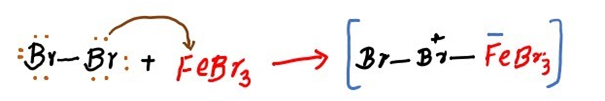
Step 2: Electrophilic attack and formation of the sigma complex.
As the bromine has now become more electrophilic after activation of a catalyst, an electrophilic attack by the benzene occurs at the terminal bromine of Br-Br-FeBr3. This allows the other bromine atom to leave with the FeBr3 as a good leaving group, FeBr4-.
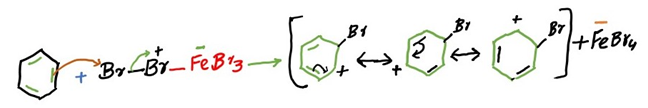
Step 3: Loss of a proton gives the products.
After the electrophilic attack of bromide on the benzene, the hydrogen on the same carbon as bromine substitutes the carbocation which resulted from the attack. Hence it is an electrophilic aromatic substitution.
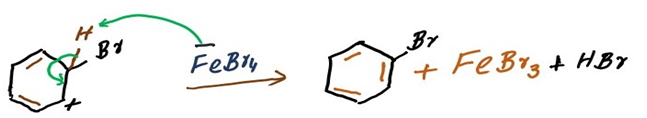
In the end, FeBr3 was not consumed by the reaction and is regenerated. It serves as our catalyst in the halogenation of benzenes.
Similarly addition of chlorine into benzene is also known as chlorination of benzene.
What is the principle of hydrogenation?
The process of hydrogenation involves hydrogen molecules splitting apart and attaching to a metal catalyst, such as nickel and platinum, as single atoms. When the unsaturated oil is introduced to the metal catalyst, the hydrogen atoms transfer to the double bond in the oil, forming new single bonds.
What is the first step of the halogenation of benzene?
A Mechanism for Halogenations of Benzene
In the first step, a strong electrophile is created to entice the pi electrons of the aromatic ring to react.
What conditions are needed for halogenations?
Energy input in the form of heat or light is necessary to initiate these halogenations. If the light is used to initiate halogenations, thousands of molecules react for each photon of light absorbed. Halogenation reactions may be conducted in either the gaseous or liquid phase.
What catalyst is needed for halogenations?
The catalyst is either aluminum chloride (or aluminum bromide if you are reacting benzene with bromine) or iron