LEARNING OBJECTIVES
In this article, author has explained the introduction, structure and broad classification of amino acids.
Definition
Table of Contents
The group of organic compounds which contain two functional groups; amino and carboxyl groups are called amino acids.
The amino group is basic while the carboxyl group is acidic in nature. The elements that are present in every amino acid are carbon, hydrogen, oxygen, and nitrogen. Amino acids are building blocks of proteins. Several amino acids are linked by a peptide bond to form a long chain of the protein.
Structure of amino acids
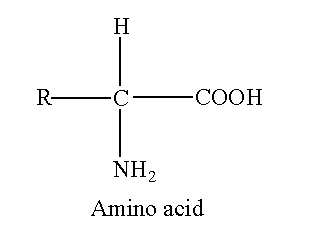
The amino acid contains a carboxyl group and amino group attached to the carbon. If amino and carboxyl group is attached to the same carbon then this carbon is called α-carbon.
When hydrogen is transferred from carboxyl to amino group, then amino acid exists as an ion.
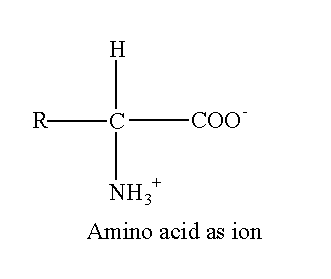
The R represents the side chain attached to the alpha carbon. Each 20 amino acids have a different side chain. Amino acids mostly exist as an ion in the biological system. If four different groups are attached to carbon, the carbon is asymmetric. The asymmetric possesses optical isomerism. All amino acids except glycine have four different groups (R, H, COO– and NH3+). So, all amino acids except glycine are optically active. The R group in glycine is H therefore glycine does not exhibit optical isomerism.
Classification of amino acids
Amino acids are classified on basis of structure, chemical nature, nutritional requirement, metabolic fate, etc.
A. Amino acid classification is based on the structure
Amino acids are classified on the base of their structure and chemical nature. Each amino acid is given 3 letters or 1 letter symbol. For example, glycine is represented by “Gly” or G. Alanine is represented by “Ala” or A
The 20 amino acids are divided into seven different groups.
1. Amino acids with aliphatic side chain
These amino acids are monoamine and monocarboxylic. For example, glycine, alanine, valine, leucine, and isoleucine are simple amino acids with an aliphatic side chain. Valine, leucine, and isoleucine contain branched aliphatic side chains. They are known as branched-chain amino acids.
2. Hydroxyl group-containing amino acids
These amino acids contain the OH group. Serine, threonine, and tyrosine are hydroxyl groups containing amino acids. Tyrosine has benzene ring. Therefore, tyrosine is also known as an aromatic amino acid.
3. Sulfur-containing amino acids
These amino acids have a sulfur group. Cysteine has a sulfhydryl group and methionine with thioether group are two amino acids included in this group. Cystine is also sulfur-containing amino acid formed by the condensation of two cysteine molecules.
4. Acidic amino acids
The amino acids containing two carboxylic groups and one amino group are acidic amino acids. In short, they are dicarboxylic monoamine acids. Aspartic acid and glutamic acid are acidic amino acids. Asparagines and glutamine are their derivatives respectively.
5. Basic amino acids
The amino acids which contain two amino and one carboxyl group are basic amino acids. They are diamino and monocarboxylic acids. Lysine, histidine, and arginine are basic amino acids. They are highly basic in nature.
6. Aromatic amino acids
The benzene ring containing amino acids is aromatic. Phenylalanine, tyrosine, and tryptophan are aromatic amino acids.
7. Imino acids
The amino acids containing imino group (NH) instead of the amino group (NH2) are imino acids. Proline is an α-imino acid. Proline contains a pyrrolidine ring.
B. Classification of amino acids based on polarity
Amino acids are divided into four classes based on polarity. Polarity plays important role in the structure of amino acids.
1. Non-polar amino acids
These amino acids are hydrophobic (water-hating). The R group present in them has no charge. Leucine, isoleucine, valine, methionine, phenylalanine, alanine, proline, and tryptophan are non-polar amino acids.
2. Polar amino acids with a neutral R group
The R group in these amino acids has no charge. These amino acids have hydroxyl, sulfhydryl, and amide groups. These groups participate in the hydrogen bonding of the proteins. Glycine, serine, threonine, cysteine, glutamine, asparagines, and tyrosine are included in this category.
3. Polar amino acids with a positive charge on the R group
Lysine, arginine, and histidine are polar and have a positive charge on the R group.
4. Polar amino acids with the negative charge on the R group
The dicarboxylic monoamine acids are included in this category. These are aspartic acid and glutamic acid.
C. Nutritional classification of amino acids
The 20 amino acids are involved in the formation of proteins. But all these proteins are not synthesized by the body. These amino acids are classified into two classes;
1. Essential amino acids
The amino acids which our body cannot synthesize are known as essential amino acids. These amino acids must b supplied through the diet. They are required for the proper growth and development of the person. The essential amino acids are valine, arginine, histidine, leucine, isoleucine, lysine, methionine, phenylalanine, threonine, and tryptophan.
There are two amino acids that can be synthesized by adults and not by children. These are known as semi-essential amino acids. The semi-essential amino acids are arginine and histidine.
2. Non-essential amino acids
The other 10 amino acids can be synthesized by the body so, they are known as non-essential amino acids. These amino acids are not needed to consume through diet. The non-essential amino acids are glycine, alanine, serine, cysteine, aspartate, asparagine, glutamine, glutamate, tyrosine, and proline.
D. Classification based on the metabolic fate
The carbon skeleton of amino acids acts as a precursor for the synthesis of glucose or fat. Amino acids are divided into three classes depending upon the metabolic fate;
1. Glycogenic amino acids
The amino acids which serve as a precursor for the synthesis of glucose or glycogen are called glycogenic amino acids.
Alanine, glycine, aspartate, and methionine are glycogenic amino acids.
2. Ketogenic amino acids
The amino acids which act as a precursor for the synthesis of fats are called ketogenic amino acids.
Leucine and lysine are ketogenic amino acids.
3. Glycogenic and ketogenic amino acids
Four amino acids are glycogenic as well as ketogenic. They act as a precursor for both glucose and fats. Phenylalanine, isoleucine, tryptophan, and tyrosine are glycogenic as well as ketogenic.
Properties of amino acids
The amino acids have the following physical properties:
- Solubility: Amino acids are soluble in water and insoluble in organic solvents.
- Melting point: Amino acids have a high melting point. They usually melt at high temperatures like above 2000C.
- Taste: Amino acids have different types of tastes. Some amino acids like glycine, alanine, and valine are sweet. Leucine is tasteless while arginine and isoleucine are bitter. Some amino acids like monosodium glutamate are used as flavoring agents in food industries. Monosodium glutamate is also in Chinese foods to increase flavor and taste.
- Optical properties: The carbon which has four different functional groups attached to it is known as asymmetric carbon. The amino acids which have asymmetric carbon are optically active. They rotate the plane with polarized light. All amino acids except glycine possess optical isomerism. The R group in glycine is H, therefore its carbon is not asymmetric.
- Amino acids as ampholytes: Amino acids have carboxylic (acidic) and amino (NH2) groups. They can donate a proton and accept the proton. Therefore, amino acids are ampholytes.
- Dipolar ion: The amino acid exists as a zwitter ion. The zwitter means hybrid. Zwitter ion is a hybrid containing both positive and negative ionic groups. The amino acid rarely exists as neutral with a free carboxylic or amino group. In strongly acidic pH, the amino acid exists as a positively charged ion while in strong alkaline pH, the amino acid exists as a negatively charged ion. Each amino acid has a specific pH at which it carries both positive and negative charges and exists as a zwitter ion.
- Isoelectric point: The pH at which amino acid carries both positive and negative charges or exists as a zwitter ion or becomes neutral is called isoelectric pH. For example, leucine exists as a cation at pH below 6 while exists as an anion at pH above 6. At pH 6, leucine exists as zwitter ion.
Reactions of amino acids
Reactions due to carboxylic group
- When amino acid reacts with bases (Na), salts (COONa) are formed.
- When amino acid reacts with alcohol, esters (COOR) are produced.
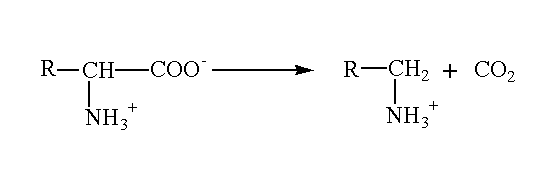
- Decarboxylation: amino acids undergo decarboxylation to produce amines. This reaction is very useful due to the formation of many biologically important amines. These include histamines, and tyramine from the amino acids histidine and tyrosine respectively.
- Reaction with ammonia: The carboxyl group of acidic amino acids reacts with NH3. This reaction produces amides.
Aspartic acid + NH3–> Asparagine
Glutamic acid + NH3–> Glutamine
Reactions due to NH2 group
- The amino groups act as bases and react with acids to form salts.
- Reaction with ninhydrin: amino acids react with ninhydrin to form the purple, pink, or blue complex.
Amino acid + Ninhydrin–> keto acid + NH3 + CO2 + Hydrindantin
Hydrindantin + NH3 + Ninhydrin–> Ruhemann’s purple
Ninhydrin is used for the quantitative determination of amino acids and proteins.
3. Color reactions of amino acids: there are specific color reactions that are used to identify amino acids. For example, millons reaction is used to identify the phenolic group of tyrosine. A sulfur test is used to indicate the sulfhydryl group of cysteine.
4. Transamination: when the amino group of amino acid is transferred to the keto acid to form new amino acid is called the transamination group.
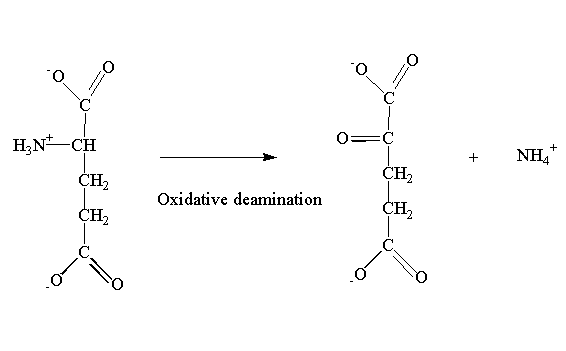
5. Oxidative deamination: amino acids undergo oxidative deamination to release ammonia.