LEARNING OBJECTIVES
In this article, the author has briefly explained the chemical bonding its types, and examples.
The force present between two atoms that hold them together and make them stable is known as a chemical bond.
Molecules of chemical substances are joined together by some force. This force is due to the interaction between the atoms of the molecule. This force is known as a chemical bond.
There are three types of chemical bonds known:
- Ionic bond or electrovalent bond
- Covalent bond
- Coordinate covalent bond
- Metallic Bonding
Before discussing the types of chemical bonding, there are some general terms you should know:
What is valence?
Table of Contents
The potential of the element to react with other elements is known as the valence or valency of the element.
Example
- In HCl, the valency of chlorine is 1.
- In MgO, the valence of Mg is 2. Some elements have different valences.
The valence can also be fractional. In short, we can say valence is the number of bonds an atom forms.
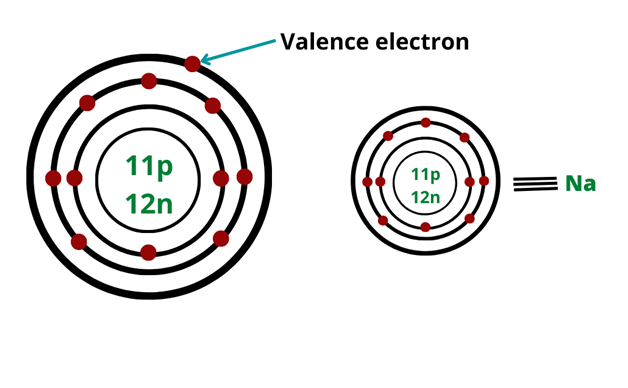
Valence electrons
The number of electrons in the outer shell of the atom that participates in the chemical bonding is known as valence electrons.
Example
- The electronic configuration of Na is 2, 8, 1. So Na has one valence electron.
- The electronic configuration of chlorine is 2, 8, and 7. Thus chlorine has seven valence electrons.
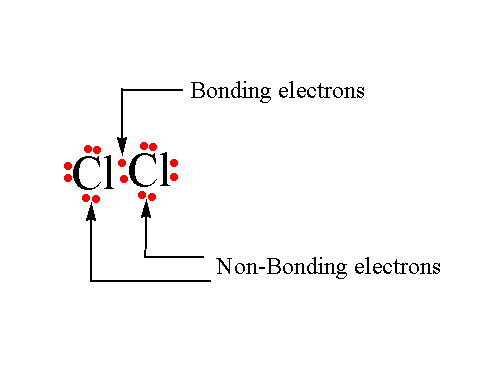
Bonding and non-bonding electrons
The valence electrons that participate in the formation of bonds are known as bonding electrons.
The remaining valence electrons that are still available for bond formation are known as non-bonding electrons.
Lewis symbol of elements
The Lewis symbol of elements represents the valence electrons. It is a diagrammatical representation of element symbols and electrons in the valence shell. The electrons are represented in the form of dots. The symbol represents the nucleus.
To represent the Lewis symbol of the element, the symbol of the element is written and dots are drawn around them. The electrons that participate in the bonding are shown at the specific position while the rest of the valence electrons are shown in pairs.

Electronic theory of valence
After Bohr’s atomic model explains the electronic configuration of elements two other Chemists G.N Lewis and W. Kossel put forward an electronic theory of valence. This theory explains why atoms join to form molecules.
The noble gases have complete octet or stable electronic configuration. All other elements have incomplete valence shells. In 1916, G.N Lewis and W. Kossel answered this question through this theory.
Atoms interact to form molecules by losing, gaining, or sharing electrons to have a stable electronic configuration or noble gas electronic configuration. All the inert gases except helium have 8 electrons in the outer shell. Helium has two electrons.
| Noble gases | At.No. | Electrons in principal shells |
| He | 2 | 2 |
| Ne | 10 | 2, 8 |
| Ar | 18 | 2, 8, 8 |
| Kr | 36 | 2, 8, 18, 8 |
| Xe | 54 | 2, 8, 18, 18, 8 |
| Rn | 86 | 2, 8, 18, 32, 18, 8 |
The tendency of an atom to have eight electrons in the valence shell is called the octet rule. The tendency of an atom to have two electrons in the valence shell is called the duplet rule. Atoms joined to form molecules to complete its octet. The octet is completed by gaining, losing, or sharing electrons.
Ionic bond
The bond formed by the complete transfer of electrons from one atom to other is called an ionic bond.
Example
Let’s discuss the general example of two atoms A and B. Atom A has one electron while atom B has 7 electrons. Atom B needed 1 electron to complete its octet and to get stable. Atom A has one electron in excess. So, A transfers the electron to B. Both the atoms get stable. When A loses an electron, it becomes a positive ion (cation) and when B accepts an electron it becomes a negative ion (anion). The opposite charges ions are held together by electrostatic attraction.
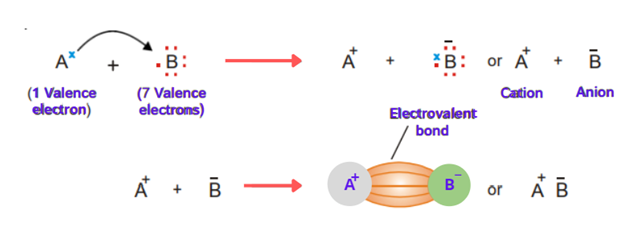
The electrostatic attraction present between cation and anion produced by the transfer of electrons is called an ionic or electrovalent bond. The compounds which have ionic bonds are called ionic compounds.
Covalent bond
The bond formed by the mutual sharing of electrons between two atoms is called a covalent bond.
Two atoms can get a stable duplet or octet by the sharing of electrons.
Example
Let’s discuss the general example of atoms A and B. A has one electron in its valence shell while atom B has seven electrons in its valence shell. When two atoms approach, they both share one electron. In this way, the duplet of A and octet of B are completed and stable.
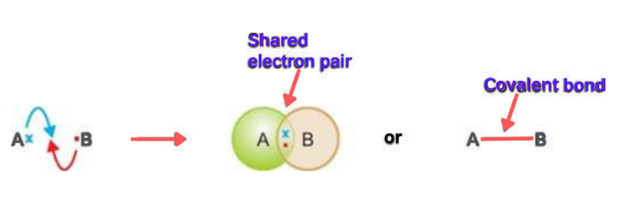
The dash (—) indicates the covalent bond which is formed by the shared pair of electrons. When electrons approach each other, attractive as well as repulsive forces start operating. A covalent bond is formed when attractive forces dominate the repulsive forces. Thus covalent bonds can also define as attractive forces between the shared pair of electrons.
The covalent bond-containing compounds are called covalent compounds.
Co-ordinate covalent bond
The covalent bond formed when pair of an electron is shared by only one atom is called a coordinate bond.
The coordinate bond is also known as the dative bond.
In the normal covalent compound, both the atoms share an electron but in a coordinate covalent bond, both electrons come from one atom only.
Example
Atom A has one lone pair and B is deficient in two electrons. A donates the lone pair to B. In this way, both atoms achieve a stable electronic configuration.
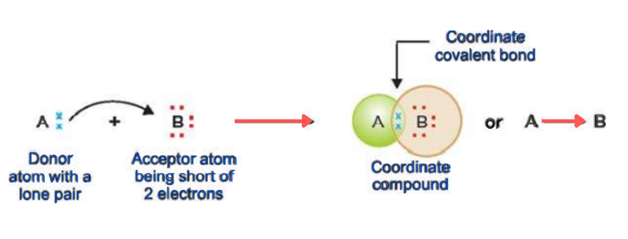
The atom which donates lone pair is known as a donor. And the atom which accepts lone pair is called the acceptor. The molecule that contains a donor atom is called a ligand.
The compounds that contain coordinate covalent bonds are called coordinate covalent compounds.
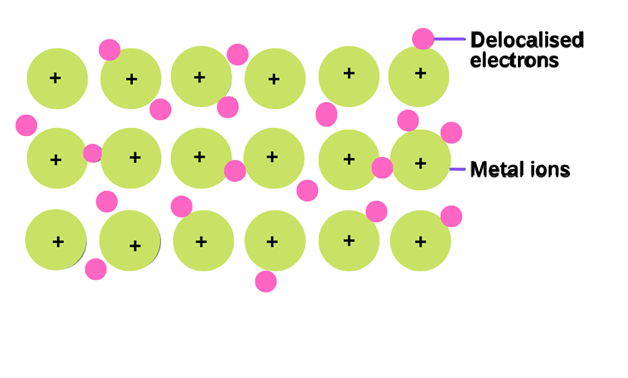
Metallic bond
The type of bonding which holds the atoms together in the metal crystal is called metallic bonding. The collective sharing of the sea of valence electrons between several positive ions is referred to as a metallic bond.
This bonding is neither ionic nor covalent. This type of bonding cannot be ionic because metals can easily lose their electrons. So all the atoms tend to lose electrons and none of them can accept it. Covalent bonding also does not work in metallic bonding. For example, sodium Na has one electron in an outer shell and cannot form a covalent bond with the 7 nearest atoms
Example
The metallic bonding is shown by sodium metal. The electronic configuration of sodium is 1s2, 2s2, 2p6, 3s1. Sodium has only one valence electron. It loses one electron and becomes the positive ion. So, the sea of valence electrons is shared between several positive ions.