Written by Adeel Abbas
What are molecules and ions in chemistry?
Table of Contents
What are Molecules in chemistry?
The smallest particle of elements or compounds that can exist independently is known to be a molecule.
Definition of Molecule
It is made up of two or more atoms of similar or different elements. Cl2, O2, NH3, C6H12O6. Anyhow, molecules of inert gases consist of a single atom.
What is Atomicity in chemistry?
The number of atoms present in a molecule refer to its atomicity.
Definition of atomicity
For example in case of methane CH4 there are 4 Hydrogen atoms and 1 carbon atom so its atomicity is 5. Similarly the atomicity of Cl2 is 2, CO2 is 3, NH3 is 4 and of C6H12O6 24.
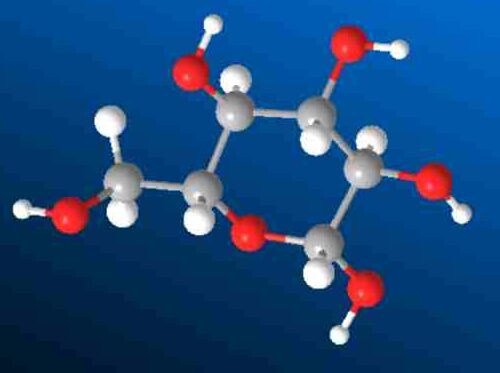
You can count the number of atoms in glucose molecule 3d structure.
Types of molecules on the basis of the number of atoms
Molecules can be categorized into multiple types on the basis of numbers of atoms present in them.
For example…
i) Monoatomic molecule
The molecules formed as a result of the combination of only one atom are known to be monoatomic molecules.
Definition of monoatomic molecule
For example, He, Ne, Ar, Kr, Xe, Sodium vapors
ii) Diatomic molecules
The molecules formed by the combination of two atoms are known to be diatomic molecules.
Definition of diatomic molecules
For example, H2, N2, O2, Cl2, Br2, HCl, HBr, CO, NO are known as diatomic molecules.
iii) Polyatomic molecules
The molecules formed by the combination of more than two atoms are known to be polyatomic molecules.
Definition of Polyatomic molecules
For example, H2O, NH3, CCl4, H2SO4, KMnO4, C6H12O6 are the examples of polyatomic molecules.
Types of molecules on the basis of type of atom
1) Homoatomic or Homonuclear diatomic molecules
The molecules formed by the combination of two same atoms are known to be homoatomic or homonuclear diatomic molecules.
Definition of homoatomic molecules
For example, in the case of H2, N2, O2, Cl2 these have been formed by the combination of two same atoms.
2) Heteronuclear diatomic molecules
Definition of heteronuclear molecules
The molecules formed by the combination of two different atoms are known to be heteronuclear diatomic molecules.
For example, in case of HF, HCl, HBr, HI, CO, NO these have been form by the combination of two different atoms.
Size of molecules
Depending on the number of atoms , molecules may be of varied sizes.
i) Molecules of substances are very small
ii) The molecules are bigger than the atoms
iii) Some of molecules are so large that they are called macromolecules
Macromolecules
The molecules of high molecular mass usually greater than 10,000 are known as macromolecules.
Definition of Macromolecules
For example…
I) Proteins ii) Haemoglobin iii) Some natural and synthetic polymers
Haemoglobin is a blood protein. It is a macromolecules. It helps to carry oxygen from our lungs to all parts of body to form oxy-haemoglobin. Each molecule consists of nearly 10,000 atoms. It is 68,000 times heavier than hydrogen atom.
What are ions in chemistry?
Ion is defined as a species that bears positive of negative charge.
Definition of ion
Types of Ions
There are following types of ions
1) Positive ion or cation
2) Negative ion or anion
1: Positive ion or Cation
A species which has positive charge is called positive ion or cation.
Cation definition
How cation is formed?
When atoms under the influence if energy lose one or more electrons, cations are formed.
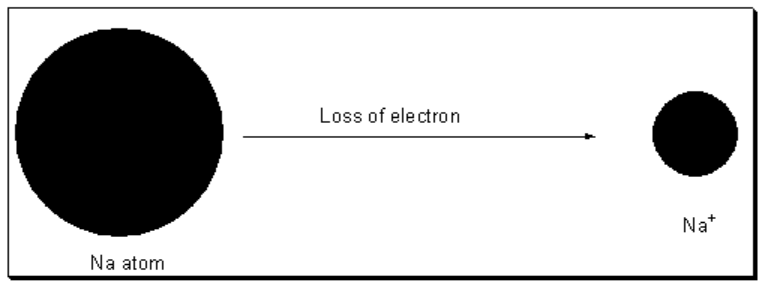
When sufficient amount of energy is supplied to the neutral atom then the atom loses electon to form positive ion.
Cations have +1, +2, +3, +4 charges
The most common and simple cations are the metal ions, such as
Na+, K+, Ca2+, Mg2+, Al3+, Fe3+, Sn4+ .
The cations having group of atom are less common e.g. NH4+, H3O+ .
Negative Ion or Anion
A species with developed negative charge is called negative ion or anion.
Anion definition
Process of anion formation?
When one or more electrons are added to an isolated neutral atom then anions are formed.
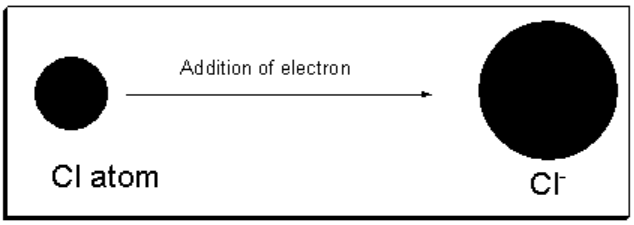
Energy is usually liberated when an electron is added to a neutral isolated atom to form a uni-negitive ion.
Anion have -1, -2, -3, -4 charge.
The common and simple anions are F–, Cl–, I–, S2-, P3- etc.
There are many examples of anions having groups of atoms like OH-, MnO4-, CO32-, PO43-, SO42-, Cr2O72- etc.
What is a Molecular ion?
Any molecular species with either positive or negative charge is said to be molecular ion.
Definition of molecular ion
Examples:
CO+, C2H4+, O2+, CH4+
Formation of the molecular ion
When beam of electron having energy 10-15eV is bombarded on a molecule of a compound in the vapour phase, an electron is removed and a radical cation is formed. This radical cation is called molecular ion.
This is done in the ionization chamber of the mass spectrometer. The fast-moving alpha particles can also ionize gas molecules to give molecular ions. A metal atom M is converted to M+ by electron bombardment.
Let others know about this