What is nitration of benzene?
Table of Contents
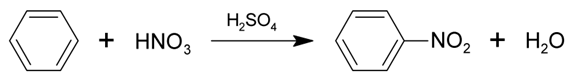
The addition of nitro (NO2) group into benzene is called nitration of benzene.
Equation
Explanation
Nitration of benzene is an example of electrophilic aromatic substitution reaction. Benzene reacts with concentrated nitric acid in the presence of sulfuric acid to give nitrobenzene.
In this reaction sulfuric acid acts as a catalyst. Catalyst is a substance which enhances the rate of chemical reaction but does not take part in chemical reaction.
Similarly other derivatives of benzene can also undergo nitration. For example nitration of toluene.
Watch the video to better understand nitration of Benzene
Nitration of benzene conditions
The nitration of benzene is a reaction that involves the substitution of a nitro group (-NO2) for a hydrogen atom on a benzene ring. The conditions required for this reaction are:
Reagents: A mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) is used as the nitrating agent. This mixture is called a nitrating mixture or nitration mixture.
Temperature: The nitration of benzene is an exothermic reaction, which means it releases heat. To control the reaction and prevent a runaway reaction, the reaction mixture should be kept at a temperature of 50-60°C.
Catalyst: No catalyst is required for this reaction as the sulfuric acid acts as a catalyst by protonating the nitric acid, making it more reactive.
Stoichiometry: The stoichiometry of the reaction is important, and the ratio of nitric acid to benzene should be carefully controlled. Generally, a 1:1.5 to 1:2 molar ratio of nitric acid to benzene is used.
The overall reaction can be represented as:
C6H6 + HNO3 + H2SO4 → C6H5NO2 + H2O
Where C6H6 is benzene, HNO3 is nitric acid, and C6H5NO2 is nitrobenzene.
Mechanism of nitration of benzene
The mechanism of nitration of benzene involves three steps which are given below.
STEP 1: Formation of nitronium ion (NO2+) from Nitric acid
The nitronium ion is formed by the reaction of nitric acid with sulfuric acid. Sulfuric acid will protonate nitric acid leading to a loss of water molecule and formation of nitronium ion. The nitronium ion is a powerful electrophile and is attacked quickly by the aromatic ring.
Remember that during this step temperature must not exceed 50 °C.
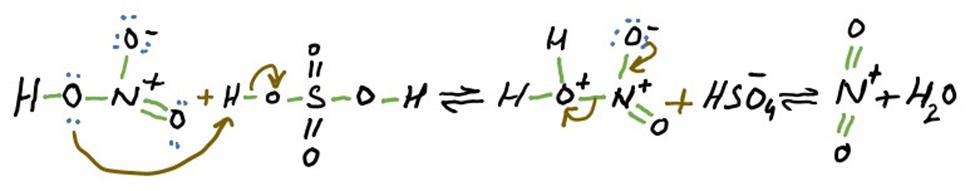
Important point in Nitration of Benzene
In this reaction both reactants are acids while proton is only denoted by sulfuric acid . First reason is that sulfuric acid is a strong acid. Second reason is that lone pair of oxygen atom that is directly bonded to sulfur atom in sulfuric acid is involved in resonance. This resonance increase the stability. On the other hand lone pair of oxygen atom directly bonded to nitrogen atom in nitric acid is available for attack on proton. This is the reason that nitric acid does not provide its electron in this reaction.
Step 2: Attack of electrophile to give arenium ion.
In this step electrophile (nitronium ion) attacks on the benzene ring which gives the formation of arenium ion. It is also known as sigma complex.
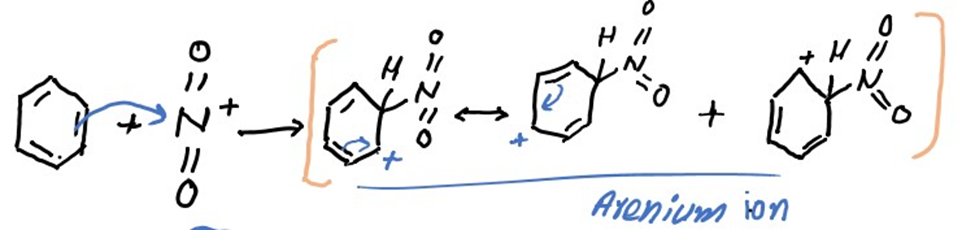
Step 3: Loss of proton to give nitrobenzene.
In this step sulphonate ion accepts proton from arenium ion which gives the nitrobenzene.

From above image sulfuric acid is also recovered that was used as a catalyst at the start of the reaction. Finally our product nitrobenzene is produced at the end of reaction.
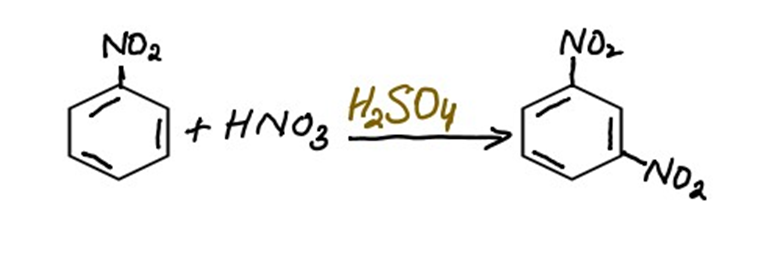
Mechanism of nitration of nitrobenzene
Overall reaction
i) Formation of electrophile
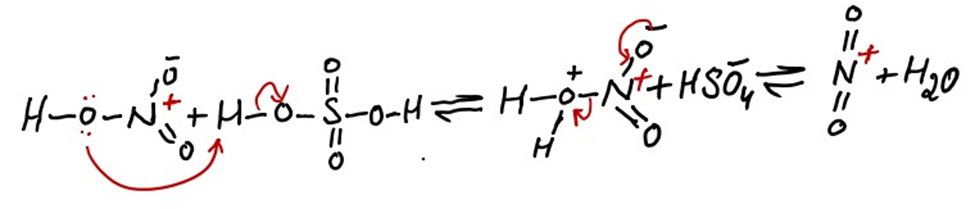
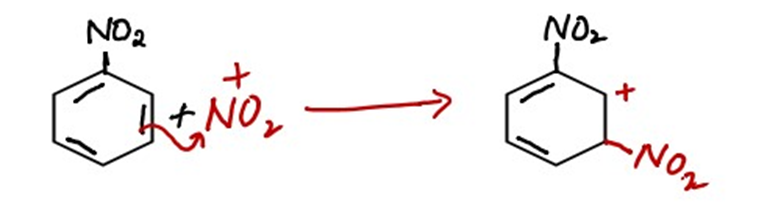
ii) Attack of electrophile
The electrophile attacks the Meta position of the benzene ring because the group which is already present on the benzene ring is an electron-withdrawing group. Electron withdrawing groups directs the incoming reagent toward the Meta position.
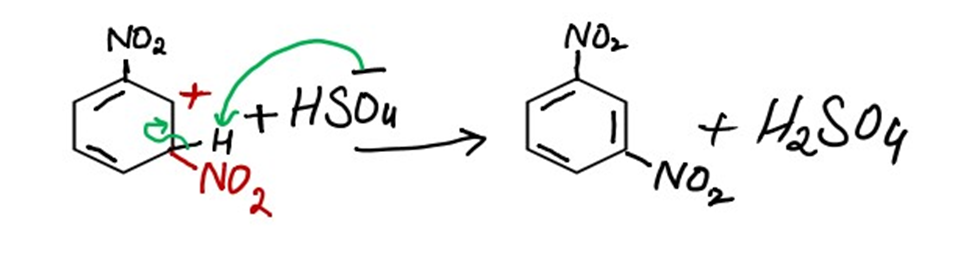
iii) Removal of the proton to regenerate aromaticity
FAQs
What is the function of concentrated sulfuric acid in the nitration of benzene?
Concentrated sulfuric acid acts as a catalyst in the nitration reaction. It speeds up the reaction by reacting with nitric acid to form the nitronium ion which is a better electrophile.
What is the catalyst used in the nitration of benzene?
Sulfuric acid is used as a catalyst in the nitration of benzene, and it forms nitronium ion which attacks the benzene ring, and nitrobenzene is formed.
Is nitration of benzene an electrophilic substitution?
It is an electrophilic substitution reaction in which a nitro group is introduced into the benzene ring when it is heated with a mixture of concentrated nitric acid and concentrated sulphuric acid.
What is electrophile in the nitration of benzene?
The electrophile is the nitration of benzene is a “nitronium ion” (NO+2). This is formed by a reaction between nitric acid and sulphuric acid.
what is produced when benzene reacts with nitric acid in the presence of sulfuric acid?
Benzene reacts with concentrated nitric acid at 50-60 °C in the presence of concentrated sulphuric acid to form nitrobenzene. This reaction is known as the nitration of benzene.