Written by Adeel Abbas
In Side-Chain Reactions of benzene derivatives, any group which is directly attached to an aryl group is replaced by another group in the presence of a strong agent.
While certain reactions depend on the aromatic ring to speed up the reaction, many are unaffected by the presence of a neighboring benzene ring. For instance, the Clemmensen reduction works best when reducing aryl ketones to alkylbenzenes, while it can also be used to reduce aliphatic ketones to alkanes. Additional side-chain reactions demonstrate the impact of an adjacent aromatic ring.
Key Points
- Side-chain reactions of benzene derivatives involve the replacement of a group directly attached to an aryl group with another group in the presence of a strong agent
- Certain reactions depend on the presence of an aromatic ring to speed up the reaction, while others are unaffected by it
- One example of a side-chain reaction is permanganate oxidation, where the alkyl side chain is oxidized while the benzene ring remains unharmed
- Another example is side-chain halogenation, where alkylbenzenes are more easily halogenated by free radicals than alkanes
- Benzylic halides are more reactive in nucleophilic substitution reactions than most alkyl halides due to the stabilization of the transition state by conjugation
- SN2 reactions of benzyl halides are particularly effective in converting aromatic methyl groups to functional groups.
Permanganate Oxidation
Table of Contents
The benzene ring is not oxidized by potassium permanganate. Under strong circumstances, however, the alkyl side chain is completely oxidized, resulting in the production of carboxylic acid at the location of the alkyl group. When a ring has two or more alkyl groups, they are all oxidized. However, the benzene ring is unharmed.
The closest carbon atom of a side chain to an aromatic ring gains more stability. An energetic permanganate oxidation can preserve the aromatic ring and one carbon atom of a side chain. The item is a benzoic acid carboxylate salt. (This oxidation may also be carried out using hot chromic acid.)
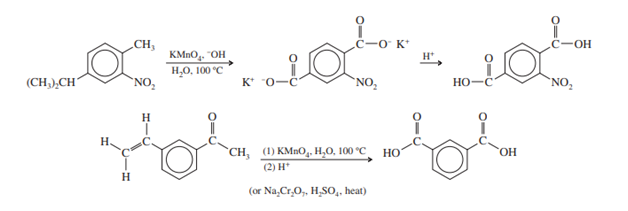
Side-Chain Halogenation
Since the abstraction of a hydrogen atom from the benzylic position results in a resonance-stabilized benzylic radical, alkylbenzenes are much more easily halogenated by free radicals than alkanes. When light and chlorine are present, for instance, ethylbenzene reacts to produce A product that can be further chlorinated to produce a dechlorinated product. However, the reaction doesn’t stop there, and all three hydrogens in the methyl group can in turn be replaced by chlorine atoms.
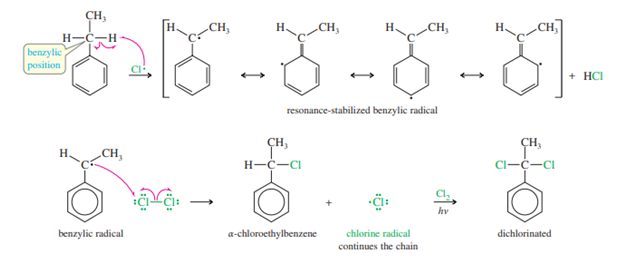
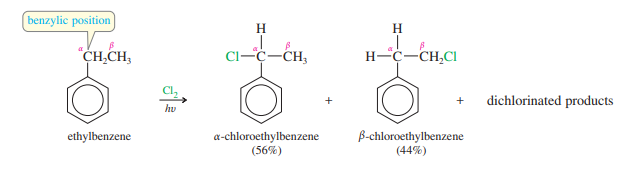
Although chlorination shows a preference for substitution (the position is the benzylic carbon bonded to the benzene ring), the chlorine radical is too reactive to give an entirely benzylic substitution. Mixtures of isomers are often produced. In the chlorination of ethylbenzene, for example, there is a significant amount of substitution at the carbon.
Bromine radicals are not as reactive as chlorine radicals, so bromination is more selective than chlorination. Bromine reacts exclusively at the benzylic position.
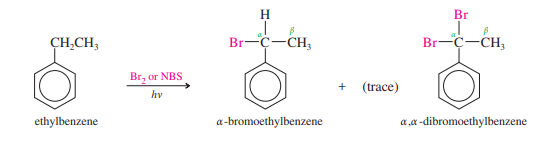
For benzylic bromination, the reagent can either be N-bromosuccinimide, which is significantly less expensive, or elemental bromine. For allylic bromination, N-bromosuccinimide is chosen because it can strengthen the double bond. Unless it possesses strong activating substituents, the comparatively inert benzene ring does not pose an issue in this situation.
Nucleophilic Substitution at the Benzylic Position
Even when mild nucleophiles are present, benzylic halides easily engage in nucleophilic substitution processes. Conjugated alkenes are easily produced by benzylic alcohols or halides. Alkenylbenznes are added to produce the usual additional products.
Allylic halides are more reactive than most alkyl halides in both SN1 and SN2 reactions. Benzylic halides are also more reactive in these substitutions, for reasons similar to those for allylic halides.
First-Order Reactions
To produce a carbocation through first-order nucleophilic substitution, the halide must be ionized. The carbocation is resonance-stabilized in the case of a benzylic halide. For instance, the 2-degree cation 1-phenylmethyl is roughly as stable as a 3-degree alkyl cation.
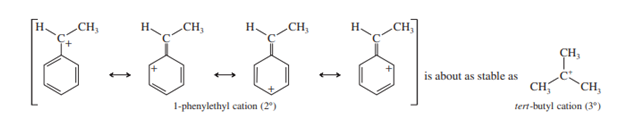
Because they form relatively stable carbocations, benzyl halides undergo reactions more easily than most alkyl halides.
The stabilizing effects are additive if more than one phenyl group is linked to a benzylic cation. The triphenylmethyl cation serves as a striking illustration. Three phenyl groups help to maintain the positive charge, making this cation particularly stable. In reality, triphenylmethyl fluoroborate is a stable ionic solid that can be kept for years in storage.
Second-Order Reactions
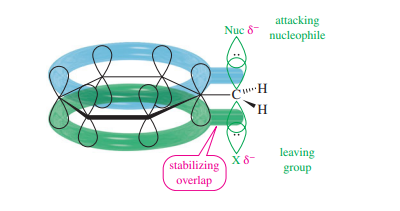
Similar to allylic halides, benzylic halides are around 100 times more reactive in displacement processes than primary alkyl halides. Similar to how allylic halides react, this increased reactivity has a reason. The p orbital partially binds with the nucleophile during displacement on a benzylic halide, and the leaving group additionally overlaps with the ring’s pi electrons. The energy of the transition state is reduced by this stabilizing conjugation, speeding up the reaction.
SN2 reactions of benzyl halides efficiently convert aromatic methyl groups to functional groups. Halogenation, followed by substitution, gives the functionalized product.
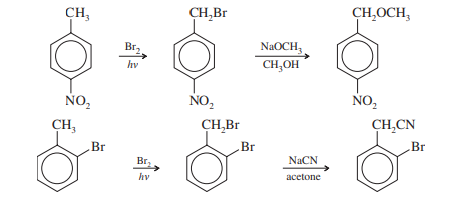
This is the final product of Nucleophilic Substitution at the Benzylic Position occupied by second-order reactions.
FAQS
What are the derivatives of benzene?
The benzene derivative is those compounds in which the benzene ring is attached to any functional group. The example of benzene derivatives are toluene, dimethylbenzene, and trimethylbenzene.
What are the characteristic reactions of benzene and its derivatives?
Benzene and its derivatives are much more stable than expected. The extra stability means that benzene will less readily undergo addition reactions. The more loosely held electrons are open to attack by electrophiles. Hence, the characteristic reaction of benzene is an electrophilic substitution reaction.
What is side chain substituted aromatic compounds give examples.
Examples include −CH2OH, −CH2OCH3, −CH2Cl, −CHO, −COCH3, −CO2H, and −CN, and we shall refer to aromatic compounds containing substituents of this type as aromatic side-chain compounds.
How are benzene derivatives formed?
The phenyl group is formed by removing one hydrogen from benzene to create the fragment C6H5. Although the molecular formula of the phenyl group is C6H5, the phenyl group would always have something attached to where the hydrogen was removed.
Why are benzene derivatives important?
Ethyl benzene is used to manufacture styrene, which is used in the production of latex, synthetic rubber, and polystyrene, which is used mainly in food packaging. Phenol and its derivatives are used to make aspirin, antiseptics, explosives, plastics, and dyes used in textiles and food.