This latest research into the dissociation of water has finally answered a crucial question. Dopant atoms or defects will stabilize polarons at the surface.
This is shown by experiments conducted at the Advanced Light Source at Lawrence Berkeley National Laboratory.
If you look at it like this, this insight might help you to think about your own design for a better, more efficient photoanode.
“Basically, we know that just by immersing bismuth vanadate in the aqueous solution, the chemical composition of the surface changes,” says Dr. Marco Favaro.
Though there have been many studies on bismuth vanadate, we do not know how much of an impact this has on electronic properties in contact with water molecules.
The research team has now investigated this question.
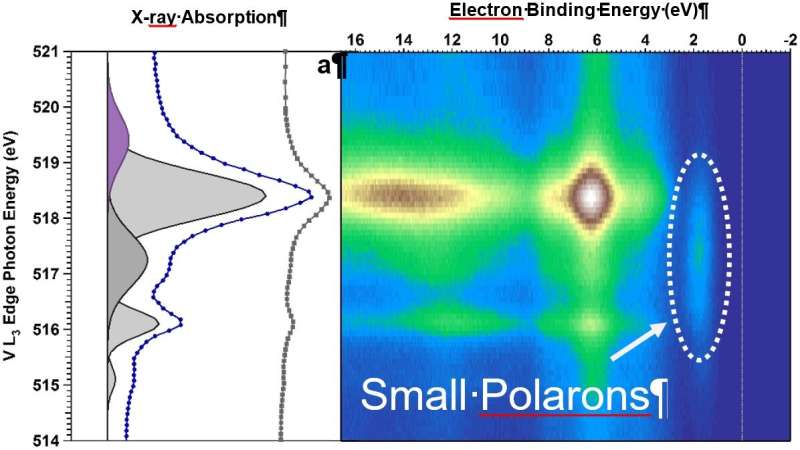
They studied single crystals of BiVO4 doped with molybdenum at the Advanced Light Source at Lawrence Berkeley National Laboratory.
Team leader and researcher at the University of Chicago, Giulia Galli, used a combination of computational and theoretical techniques to understand the experimental results.
We haven’t been able to determine if polarons play a major role in charge transfer or not.
Even if a business needs little improvement and is very efficient, they should look for ways to improve their efficiency and see what obstacles they need to remove, says Starr.
Results show that the photoanode performance is influenced by surface chemistry. Understanding the influence of the surface chemical composition of the photoanode materials is important for the design of efficient solar energy conversion systems.
Read Further information
Wennie Wang et al, Influence of Excess Charge on Water Adsorption on the BiVO4(010) Surface, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c07501.
Read More Latest Chemistry Research News
- Scientists discover unique peptides with anti-cancer potential
- New Study Shows Nanocrystals store light energy and drive chemical reactions
- Chemists create an ‘artificial photosynthesis system ten times better than existing systems
- Beer hops compounds could help protect against Alzheimer’s disease
- Scientists found method for extending shelf life of fresh pasta by 30 days