The key difference between a titrant and a titrand is their role in titration. The titrant, a known-concentration solution, reacts with the titrand, an unknown-concentration solution. The goal is to measure the titrant volume needed for the reaction to reach equivalence, allowing the calculation of the titrand’s unknown concentration. The known concentration of the titrant is crucial for determining the unknown concentration of the titrand.
What is a Titrant?
Table of Contents
The titrant is the solution that is added to the titrand in a titration experiment.
Titrant definition
It is typically a strong acid or base, and its concentration is known. The titrant is usually added to the titrand using a burette, which is a long, narrow tube with gradations that allow the volume of the titrant to be accurately measured.
What is a Titrand?
The titrand is the solution that is being analyzed in a titration experiment.
Titrand definition
Its concentration is unknown and is being determined through the use of the titrant. The titrand is typically an acid or base, but it could also be a salt or other compound that can be neutralized by the titrant.
Examples
Here are a few examples of titration experiments that illustrate the difference between the titrant and the titrand:
- In a titration to determine the concentration of hydrochloric acid in a solution, the hydrochloric acid solution would be the titrand, and a sodium hydroxide solution would be the titrant.
- In a titration to determine the concentration of a protein in a solution, the protein solution would be the titrand, and a solution of a known concentration of an amino acid would be the titrant.
- In a titration to determine the concentration of a metal ion in a solution, the metal ion solution would be the titrand, and a solution of a known concentration of a ligand would be the titrant.
Difference between titrant and titrand
The key differences between the titrant and the titrand as as follows:
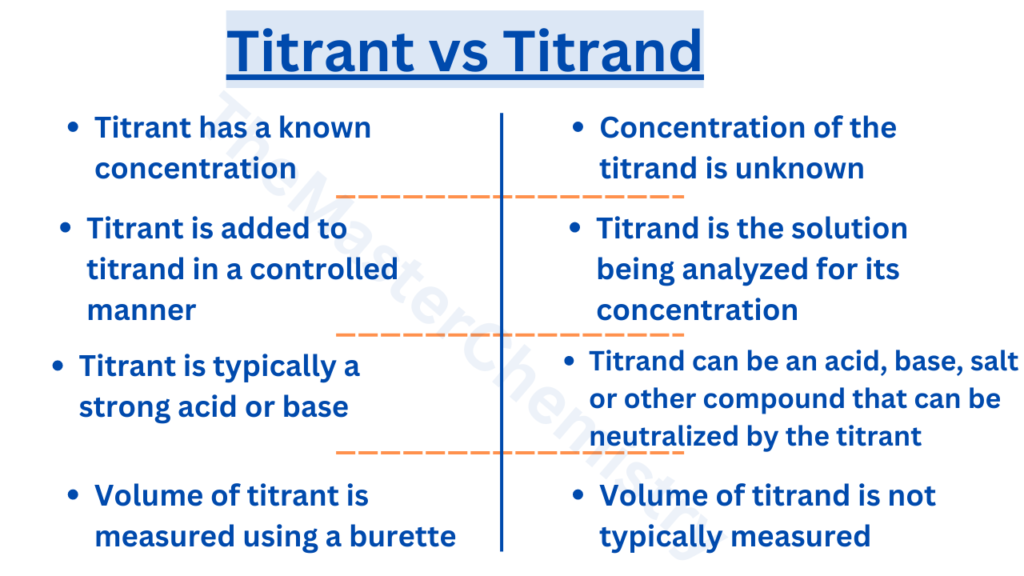
Concentration: The titrant has a known concentration, while the concentration of the titrand is unknown and is being determined through the use of the titrant.
Role in the experiment: The titrant is added to the titrand in a controlled manner, while the titrand is the solution being analyzed and its concentration is being determined.
Types of solutions: The titrant is typically a strong acid or base, while the titrand can be an acid, base, salt, or other compound that can be neutralized by the titrant.
Measuring volume: The volume of the titrant is usually measured using a burette, while the volume of the titrand is not typically measured.
Endpoint: The endpoint of a titration is the point at which the reaction between the titrant and the titrand is complete, and it is typically determined using an indicator or a pH meter.
Here is a summary of the differences between the titrant and the titrand in table format:
| Property | Titrant | Titrand |
| Concentration | Known | Unknown |
| Role in experiment | Solution added to titrand | Solution being analyzed |
| Types of solutions | Strong acid or base | Acid, base, salt, or other |
| Measuring volume | Yes (using a burette) | No |
| Endpoint | Determined using indicator or pH meter | N/A |
In summary, the titrant is the solution with the known concentration that is added to the titrand, the solution with the unknown concentration, in a titration experiment. Understanding the difference between the titrant and the titrand is essential for correctly performing and interpreting the results of a titration experiment.