Written by Adeel Abbas
What is Triple Point in Chemistry?
Table of Contents
Triple point, in thermodynamics, is the temperature and pressure at which three phases (gas, liquid, and solid) of a substance coexist in thermodynamic equilibrium.
In other words, at the triple point, the three phases of a substance can coexist without any change in temperature or pressure.
Key points
- Triple point is the temperature and pressure at which all states of matter (except plasma) coexist in equilibrium.
- It defines a substance’s phase behavior and is unique to each substance based on composition and molecular structure.
- Important in thermodynamics, phase diagrams, phase transitions, refrigeration, and heat transfer.
- Determined using a phase diagram by measuring phase change temperature and pressure.
- Examples: Water triple point is 0.01°C and 611.73 Pa; CO2 is -56.6°C and 5.18 bar.
- Phase diagrams graph temperature, pressure, and phase relationships and are used to determine triple point and predict substance behavior.
Definition of Triple Point
Triple point of a substance is defined as the temperature and pressure at which the three phases of the substance are in equilibrium. This is an important concept in thermodynamics and is used to define the phase behavior of a substance. The triple point is unique to each substance and is dependent on the chemical composition and molecular structure of the substance.
Importance of Triple Point in Chemistry
The triple point is an important concept in thermodynamics and is used to define the phase behavior of a substance. It is used in many areas of chemistry, including phase diagrams, thermodynamics, and phase transitions. The triple point is also used in engineering and technology, including the design and operation of refrigeration and heat transfer systems.
How to Determine the Triple Point of a Substance?
The triple point of a substance can be determined experimentally by using a phase diagram. A phase diagram is a graphical representation of the relationships between temperature, pressure, and the phases of a substance. The phase diagram for a substance can be obtained by measuring the temperature and pressure at which the substance changes from one phase to another.
Triple Point of Water
Water is a well-known substance and is used as a reference for the triple point in thermodynamics. The triple point of water is defined as the temperature and pressure at which the three phases of water coexist in thermodynamic equilibrium. The triple point of water is 0.01°C and 611.73 Pa.
Triple point of co2
The triple point of CO2 is the temperature and pressure at which the three phases of carbon dioxide (gas, liquid, and solid) coexist in thermodynamic equilibrium. It occurs at a temperature of -56.6 °C and a pressure of 5.18 bar.
Phase Diagrams and the Triple Point
Phase diagrams are graphical representations of the relationships between temperature, pressure, and the phases of a substance. The phase diagram for a substance can be used to determine the triple point of the substance. The phase diagram is also used to predict the behavior of a substance under different conditions of temperature and pressure.
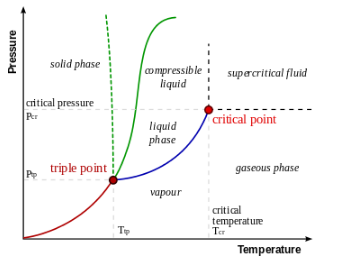
In conclusion, triple point in chemistry is an important concept that defines the temperature and pressure at which the three phases of a substance coexist in thermodynamic equilibrium. The triple point is unique to each substance and is dependent on the chemical composition and molecular structure of the substance. Understanding the concept of triple point is important in many areas of chemistry and technology, including phase diagrams, thermodynamics, and phase transitions.