The amount of product formed during a chemical reaction is called yield.
What is Yield in Chemistry?
Table of Contents
Yield is a measure of the amount of product formed in a chemical reaction, relative to the amount of reactant consumed.
It is usually expressed as a percentage. A high yield indicates that a large amount of product was formed, while a low yield indicates that a small amount of product was formed.
Types of Yield
There are two types of yield which are different from each other.
1. Theoretical yield
Theoretical yield is the maximum possible amount of a product that can be formed in a chemical reaction. It is calculated from the balanced chemical equation and the mass and relative formula mass of the limiting reactant.
The theoretical yield is a useful way to measure the efficiency of a chemical reaction.
Example of Theoretical yield
For example, let’s consider the following chemical reaction:

This reaction produces 2 moles of water for every 2 moles of hydrogen gas that react. If we start with 2 moles of hydrogen gas, the theoretical yield of water would be 2 moles.
2. Actual Yield
Actual yield is the amount of product that is actually obtained from a chemical reaction. It is usually expressed as a percentage of the theoretical yield.
The actual yield is determined by a number of factors, including the purity of the reactants, the efficiency of the reaction conditions, and the skill of the chemist. In general, the actual yield is less than the theoretical yield due to losses that occur during the reaction.
Example of Actual yield
For example, let’s consider the same chemical reaction::
2 H2 + O2 → 2 H2OThe theoretical yield of water for this reaction is 2 moles.
However, if the reaction is not carried out under ideal conditions, the actual yield may be less than 2 moles. For example, if the reactants are not pure, the actual yield will be less than 2 moles.
Percentage yield is a measure of how much of a desired product is obtained from a chemical reaction.
Percentage Yield
It is calculated by dividing the actual yield (the amount of product produced in the reaction) by the theoretical yield (the maximum amount of product that could be produced, based on the stoichiometry of the reaction) and multiplying by 100%.
Example of Percentage yield
For example, if the actual yield of a reaction is 10 grams and the theoretical yield is 15 grams, then the percentage yield would be 66.7%.
The percentage yield is also known as the efficiency of the reaction.
Percentage yield can be calculated by using this formula:
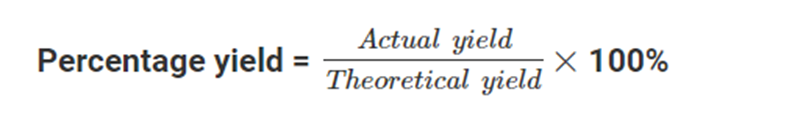
Internal standard yield
Internal standard yield is a method of calculating yield by measuring the amount of product formed (typically in the crude, unpurified reaction mixture) relative to a known amount of an added internal standard. The internal standard is a compound that is added to the reaction mixture in a known amount. It is chosen to be a compound that is not produced by the reaction, and that has similar chemical properties to the product.
The amount of product is then determined by measuring the ratio of the peak areas of the product and the internal standard in a chromatogram or spectrum. The yield is calculated as follows:
Yield = (Peak area of product)/(Peak area of internal standard) * 100%
Internal standard yield is a useful method for calculating yield when it is difficult or impossible to isolate the product. It is also useful for comparing the yields of different reactions that produce the same product.
Why Actual Yield Is Less Than Its Theoretical Yield?
Actual yield of a chemical reaction is always less than its theoretical yield. Here are the factors that can affect the actual yield of a chemical reaction:
- Incomplete reactions are one of the major reasons the actual yield is less than theoretical. Not all of the reactants react to form the desired product, which reduces the overall yield.
- Side reactions are another major reason the actual yield is less than theoretical. These are unintended reactions that result in the formation of different products. These products can reduce the amount of the desired product in the final mixture.
- Losses during separation and purification are another factor that can reduce the actual yield. Some product may be lost during these processes, such as when the product is filtered or distilled.
- Experimental errors can also contribute to a lower actual yield. These errors can include spills, contamination, and inaccurate measurements.
- Inexperienced workers may waste a significant amount of product. This can happen due to spills, contamination, and inaccurate measurements.
- Reversible reactions are never completed, so some of the reactants will always be present in the product mixture. This reduces the overall yield of the desired product.