What is boiling point?
Table of Contents
Boiling point is defined as the temperature at which vapor pressure of a liquid becomes equal to the external pressure surrounding it. This means that when the external pressure is lower, boiling points are also lower and vice versa. Vapor pressure of a liquid depends on its temperature. So if you boil water at different temperatures, its vapor pressure changes.
Some people think that boiling point is the temperature at which you can see small bubbles of vapor on the surface of water, but this isn’t exactly correct. The fact is that in a laboratory, you can create a lower pressure than the atmospheric pressure and thus the boiling point is actually below 100 °C (212 °F).
The boiling point for water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. This article goes over what boiling points mean in more detail!
| Liquids | B.P 0C |
| Acetic acid | 118.50 0C |
| Acetone | 56.00 0C |
| Aniline | 184.4 0C |
| Benzene | 80.15 0C |
| Carbon disulphide | 46.30 0C |
| Carbon tetrachloride | 76.50 0C |
| Ethanol | 78.26 0C |
| Naphthalene | 218 0C |
| Phenol | 181.80 0C |
| Water | 100 0C |
Why do we need a boiling point?
Boiling points are important for what we do every day. They allow us to cook food and sterilize medical items like Band-Aids and syringes. The boiling point also helps regulate what happens to the chemical compounds we use every day. For example, what would happen if water boiled at a temperature lower than 212 degrees Fahrenheit?
How does the boiling point affect our daily lives?
The boiling point is what happens when a liquid turns into its gas form. This has huge implications for the world around us, as it can change how we cook our food and what chemicals are used in manufacturing everything from plastic to medicine.
Effects of a higher or lower boiling point
The boiling point of a liquid is what we consider the temperature that it reaches to become a gas. The higher or lower your liquids’ boiling points are, the more likely they will evaporate and cause problems in certain applications.
For example: when water boils at 212°F (100°C), it’s not going to be an issue. You can put your hand in it for a bit and be just fine, but what if the boiling point is lower? For example: what about water at 150°F (65.55°C)? Or what about water at 100°F (38°C), this would make things like showering or cooking difficult because you will not feel warm, but what about making tea?
Factors affecting Boiling point
The boiling point of a substance depends on a lot of factors such as humidity, atmospheric pressure and even altitude. For example, water boils at 100 °C (212 °F) at sea level, but at 93.4 °C (200.1 °F) at 1,905 meters (6,250 ft) altitude.
Effect of temperature on Boiling point
The vapor pressure of a liquid increases by increasing temperature. if the temperature is increased, the vapor pressure also increases. However, if we increase external pressure then the boiling point can also be increased. Because liquids boil at temperature when their vapor pressures are equal to 760 torrs at sea level.
Strength of intermolecular forces on boiling point
Intermolecular forces are the attractive or repulsive forces between molecules. The boiling point of a liquid is directly proportional to its molecular weight and inversely proportional to van der Waals volume, which means that larger molecules boil at a lower temperature because they have weaker intermolecular force strengths.
If the intermolecular forces are stronger then vapor pressure will be lower and the boiling point will be higher. Water has a higher boiling point due to stronger intermolecular forces such as hydrogen bonding.
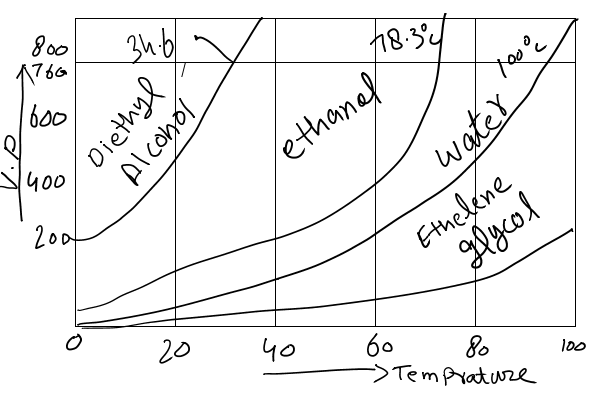
The following graph will make it clear that how intermolecular forces effects the temperature.
FAQs about boiling point
Q: How to measure boiling point?
A: If you want to know the exact temperature of boiling water, you need a device known as a boiling point apparatus. This device contains a manometer, which is used to determine vapor pressure, and a thermometer which measures the temperature of the liquid. When you heat up the water in your flask, its temperature increases at first and when it reaches 100 °C (212°F), small bubbles start to appear on the surface of the water. At this moment, vapor pressure equals atmospheric pressure and thus we say that boiling point is reached. So overall, in order to measure boiling point in an accurate way, we need two devices: one for measuring temperature and one for measuring vapor pressure
Q: What is the boiling point of water?
A: The boiling point of water is 100 °C (212 °F) at sea level, but it decreases as atmospheric pressure decreases. For example, at an altitude of 1,905 metres (6,250 ft), the boiling point of water is 93.4 °C (200.1 °F).
Q: How to decrease boiling point?
A: The boiling point of a liquid can be decreased by decreasing the external pressure surrounding it. This can be done by either increasing the atmospheric pressure or decreasing the humidity in the air. For example, if you want to boil water at a temperature lower than 100°C, you can increase the atmospheric pressure by using a pressure cooker.
Q: How to increase boiling point?
A: The boiling point of a liquid can be increased by increasing the external pressure surrounding it. This can be done by either decreasing the atmospheric pressure or increasing the humidity in the air. For example, if you want to boil water at a temperature higher than 100°C, you can decrease the atmospheric pressure by using a vacuum chamber.