LEARNING OBJECTIVES
In this article, the author has explained electronic configuration of elements in periodic table, Aufbau principle, Pauli exclusion rule, Hund’s rule with electronic configuration of all elements of periodic table.
The Electronic configuration of elements in the periodic table is a fundamental property of an element. Electronic configurations can vary based on the number of electrons and protons within an atom, which determines how many shells there are.
We will discuss Electronic configuration in detail below as relate it to periodic trends such as atomic radius, ionization energy, electron affinity, electronegativity and boiling point.
These different properties come into play when considering electronic configurations because they determine what type of chemical bonds form between atoms with different Electronic configurations – for example whether or not covalent bonds can form at all!
What are the electronic configurations of elements in the periodic table?
Table of Contents
Electronic Configurations are determined by the number of electrons within an atom’s orbitals and their relative energy levels. The shells are occupied one level at a time; first shell (K), second shell (L), third shell (M) etcetera until you reach nine where there is no further Shells. As each new shell is filled, electrons will drop to the next lower energy level.
Why is it important to know how electrons are distributed in an atom for chemistry purposes?
Electrons are the backbone of all chemistry. If you don’t understand how they are distributed in atom, then understanding any other concept in this subject is difficult. It’s important to know where an electron might be found within an atom so as not to confuse it with another element when analyzing a compound or writing chemical formulas
Electrons in an atom are distributed according to the electrons’ stability. Usually, atoms will have one or two unpaired electrons. The distribution of these unpaired electrons is called electron configuration and each type of arrangement has a name:
The most stable arrangement for atomic orbitals is “a bonding orbital” where there’s not any net negative charge; this is when the number of electrons in bonding orbitals equals the number of protons.
The next stable configuration for electron distribution would be a “nonbonding orbital” where there’s not any net positive charge, then an antibonding orbital that has more electrons than protons and finally a lone pair with one unpaired electron on either end.
The electrons in an atom are considered more stable when they’re “bonding orbitals” because this is where there’s no net charge and the electron distribution is symmetrical; if you had to choose between bonding or antibonding, it would always be better for the electrons to be in a bonding orbital.
This doesn’t mean that the electrons will be in a “bonding orbital” the entire time. Electrons can jump between orbitals by absorbing energy from outside sources that’s either transmitted (photon) or absorbed, then released into an orbital of lower potential.
This jumping process is called quantum tunneling and it occurs when there are no other places for the electron to jump to and it’s not stable in the current orbital.
The number of electrons that are distributed between orbitals will determine how many different types of atoms there are, which will also affect chemists’ ability to predict what type of chemical reactions might occur when two or more elements come into contact with one another.
The importance of understanding electron configurations and their effect on chemical properties
Electron configurations are a very important factor in determining the chemical properties of an atom. The outermost electrons around an atom determine where it can and cannot form bonds with other atoms, which determines its stability as well as what kind of reactions it will undergo. For example:
A noble gas like helium has a full shell so it does not have any bonding ability or affinity for forming new compounds. It is highly unreactive and widely used in balloons because it doesn’t cause them to explode when heated due to this lack of reactivity.
The element lithium has a full shell as well but with only two electrons, so it can form bonds and tends to be more reactive than helium. It is commonly used in rechargeable batteries due to its reactivity.
The element boron also has a full orbital of eight electrons and so does not have any bonding ability or affinity for forming new compounds like the noble gas helium; however, it is quite reactive because one electron on each side of the atom are free from being involved in chemical reactions. Boron reacts poorly when exposed to water vapor at high temperatures which will create an oxide coating that prevents further reaction between elements- this type of reaction is called catalysis.
The element oxygen has a full shell with eight electrons, but it can form bonds because of the two free electrons on each side. Oxygen is one of the most reactive elements- that’s why we put people to sleep before surgery using an oxygen mask. It reacts poorly when exposed to water vapor at high temperatures which will create an oxide coating that prevents further reaction between elements and this type of reaction is called catalysis.
Therefore electron configurations play a major role in determining a chemical’s reactivity and what kind of reactions it is capable of.
How to write Electronic Configurations?
Shells
The maximum number of electrons that can be accommodated in a shell is based on the principal quantum number (n). This formula 2n2, where ‘n’ is the shell level. The shells and values for n have been tabulated below:
| Shell and ‘n’ value | Max. Electrons in the Electron Configuration |
| K shell, n=1 | 2*12 = 2 |
| L shell, n=2 | 2*22 = 8 |
| M shell, n=3 | 2*32 = 18 |
| N shell, n=4 | 2*42 = 32 |
Subshells
Electrons are distributed into subshells depending on their azimuthal quantum number (denoted by ‘l’). This quantum number is dependent upon the value of the principal quantum, n. When n has a value of 4, four different subshells can exist with electrons occupying l=0, 1 , 2 and 3 respectively. The s-subshell accommodates up to 2 electrons; p-subshell: 6; d-subshell contains 10 while f does not have any restrictions in this regard accommodating 14 total or two less than its maximum occupancy capacity as given by the formula max =2*(2l+1) .
All the possible subshells for values of n up to 4 are tabulated below.
| Principle Quantum Number Value | Value of Azimuthal Quantum Number | Resulting Subshell in the Electron Configuration |
| n=1 | l=0 | 1s |
| n=2 | l=0 | 2s |
| l=1 | 2p | |
| n=3 | l=0 | 3s |
| l=1 | 3p | |
| l=2 | 3d | |
| n=4 | l=0 | 4s |
| l=1 | 4p | |
| l=2 | 4d | |
| l=3 | 4f |
1p, 2d and 3f orbitals are not really considered to exist because the quantum number for these orbital is always less than that of a principal quantum.
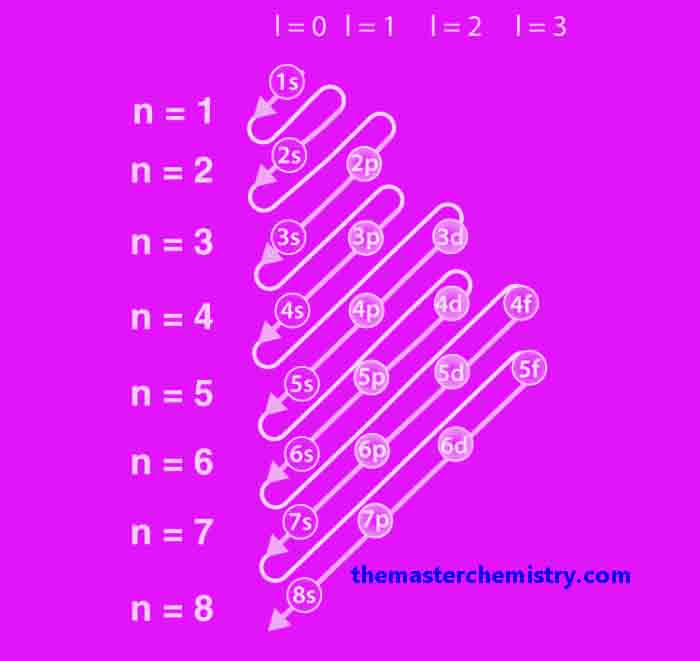
Aufbau principle and filling of atomic orbitals
The Aufbau principle, is the idea that electrons occupy orbitals of lower energy before occupying higher-energy orbitals. Electrons are filled in order from 1s to 7p and an illustration can be seen below:
Pauli Exclusion Principle and filling of atomic orbitals
In 1931, Wolfgang Pauli postulated an important principle to limit the number of electrons in a single orbital. He found that only two can occupy this space at any given time and they must be oppositely spin for it not to create instability in atoms with more than one electron.
This is also known as his “Principle of No More Than Two Electrons”. The idea behind this was what if there were too many? It would cause extreme chaos and lack stability which could lead to chemical reactions depending on how strong their magnetic fields are (since all electrons have slight magnets).
Hund’s Rule and filling of atomic orbitals
Hund’s rule is a quantum mechanical rule that determines the order in which electrons are filled. It states that every orbital in a given subshell must be singly occupied by an electron before another one enters, and it explains how to maximize total spin of all these electrons.
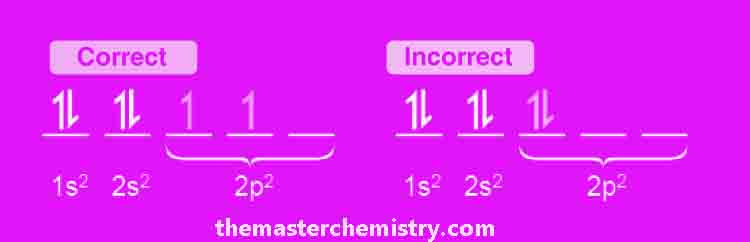
Following diagram explains the Hund’s rule.
Electronic configuration of elements in the periodic table
The electronic configuration chart given below contains all the elements with their electronic configuration.
Electronic configuration chart
| Atomic Number | Name of element | Electronic configuration |
| 1 | Hydrogen | 1s1 |
| 2 | Helium | 1s2 |
| 3 | Lithium | [He]2s1 |
| 4 | Beryllium | [He]2s2 |
| 5 | Boron | [He]2s22p1 |
| 6 | Carbon | [He]2s22p2 |
| 7 | Nitrogen | [He]2s22p3 |
| 8 | Oxygen | [He]2s22p4 |
| 9 | Fluorine | [He]2s22p5 |
| 10 | Neon | [He]2s22p6 |
| 11 | Sodium | [Ne]3s1 |
| 12 | Magnesium | [Ne]3s2 |
| 13 | Aluminum | [Ne]3s23p1 |
| 14 | Silicon | [Ne]3s23p2 |
| 15 | Phosphorus | [Ne]3s23p3 |
| 16 | Sulfur | [Ne]3s23p4 |
| 17 | Chlorine | [Ne]3s23p5 |
| 18 | Argon | [Ne]3s23p6 |
| 19 | Potassium | [Ar]4s1 |
| 20 | Calcium | [Ar]4s2 |
| 21 | Scandium | [Ar]3d14s2 |
| 22 | Titanium | [Ar]3d24s2 |
| 23 | Vanadium | [Ar]3d34s2 |
| 24 | Chromium | [Ar]3d54s1 |
| 25 | Manganese | [Ar]3d54s2 |
| 26 | Iron | [Ar]3d64s2 |
| 27 | Cobalt | [Ar]3d74s2 |
| 28 | Nickel | [Ar]3d84s2 |
| 29 | Copper | [Ar]3d104s1 |
| 30 | Zinc | [Ar]3d104s2 |
| 31 | Gallium | [Ar]3d104s24p1 |
| 32 | Germanium | [Ar]3d104s24p2 |
| 33 | Arsenic | [Ar]3d104s24p3 |
| 34 | Selenium | [Ar]3d104s24p4 |
| 35 | Bromine | [Ar]3d104s24p5 |
| 36 | Krypton | [Ar]3d104s24p6 |
| 37 | Rubidium | [Kr]5s1 |
| 38 | Strontium | [Kr]5s2 |
| 39 | Yttrium | [Kr]4d15s2 |
| 40 | Zirconium | [Kr]4d25s2 |
| 41 | Niobium | [Kr]4d45s1 |
| 42 | Molybdenum | [Kr]4d55s1 |
| 43 | Technetium | [Kr]4d55s2 |
| 44 | Ruthenium | [Kr]4d75s1 |
| 45 | Rhodium | [Kr]4d85s1 |
| 46 | Palladium | [Kr]4d10 |
| 47 | Silver | [Kr]4d105s1 |
| 48 | Cadmium | [Kr]4d105s2 |
| 49 | Indium | [Kr]4d105s25p1 |
| 50 | Tin | [Kr]4d105s25p2 |
| 51 | Antimony | [Kr]4d105s25p3 |
| 52 | Tellurium | [Kr]4d105s25p4 |
| 53 | Iodine | [Kr]4d105s25p5 |
| 54 | Xenon | [Kr]4d105s25p6 |
| 55 | Cesium | [Xe]6s1 |
| 56 | Barium | [Xe]6s2 |
| 57 | Lanthanum | [Xe]5d16s2 |
| 58 | Cerium | [Xe]4f15d16s2 |
| 59 | Praseodymium | [Xe]4f36s2 |
| 60 | Neodymium | [Xe]4f46s2 |
| 61 | Promethium | [Xe]4f56s2 |
| 62 | Samarium | [Xe]4f66s2 |
| 63 | Europium | [Xe]4f76s2 |
| 64 | Gadolinium | [Xe]4f75d16s2 |
| 65 | Terbium | [Xe]4f96s2 |
| 66 | Dysprosium | [Xe]4f106s2 |
| 67 | Holmium | [Xe]4f116s2 |
| 68 | Erbium | [Xe]4f126s2 |
| 69 | Thulium | [Xe]4f136s2 |
| 70 | Ytterbium | [Xe]4f146s2 |
| 71 | Lutetium | [Xe]4f145d16s2 |
| 72 | Hafnium | [Xe]4f145d26s2 |
| 73 | Tantalum | [Xe]4f145d36s2 |
| 74 | Tungsten | [Xe]4f145d46s2 |
| 75 | Rhenium | [Xe]4f145d56s2 |
| 76 | Osmium | [Xe]4f145d66s2 |
| 77 | Iridium | [Xe]4f145d76s2 |
| 78 | Platinum | [Xe]4f145d96s1 |
| 79 | Gold | [Xe]4f145d106s1 |
| 80 | Mercury | [Xe]4f145d106s2 |
| 81 | Thallium | [Xe]4f145d106s26p1 |
| 82 | Lead | [Xe]4f145d106s26p2 |
| 83 | Bismuth | [Xe]4f145d106s26p3 |
| 84 | Polonium | [Xe]4f145d106s26p4 |
| 85 | Astatine | [Xe]4f145d106s26p5 |
| 86 | Radon | [Xe]4f145d106s26p6 |
| 87 | Francium | [Rn]7s1 |
| 88 | Radium | [Rn]7s2 |
| 89 | Actinium | [Rn]6d17s2 |
| 90 | Thorium | [Rn]6d27s2 |
| 91 | Protactinium | [Rn]5f26d17s2 |
| 92 | Uranium | [Rn]5f36d17s2 |
| 93 | Neptunium | [Rn]5f46d17s2 |
| 94 | Plutonium | [Rn]5f67s2 |
| 95 | Americium | [Rn]5f77s2 |
| 96 | Curium | [Rn]5f76d17s2 |
| 97 | Berkelium | [Rn]5f97s2 |
| 98 | Californium | [Rn]5f107s2 |
| 99 | Einsteinium | [Rn]5f117s2 |
| 100 | Fermium | [Rn]5f127s2 |
| 101 | Mendelevium | [Rn]5f137s2 |
| 102 | Nobelium | [Rn]5f147s2 |
| 103 | Lawrencium | [Rn]5f147s27p1 |
| 104 | Rutherfordium | [Rn]5f146d27s2 |
| 105 | Dubnium | *[Rn]5f146d37s2 |
| 106 | Seaborgium | *[Rn]5f146d47s2 |
| 107 | Bohrium | *[Rn]5f146d57s2 |
| 108 | Hassium | *[Rn]5f146d67s2 |
| 109 | Meitnerium | *[Rn]5f146d77s2 |
| 110 | Darmstadtium | *[Rn]5f146d97s1 |
| 111 | Roentgenium | *[Rn]5f146d107s1 |
| 112 | Copernium | *[Rn]5f146d107s2 |
| 113 | Nihonium | *[Rn]5f146d107s27p1 |
| 114 | Flerovium | *[Rn]5f146d107s27p2 |
| 115 | Moscovium | *[Rn]5f146d107s27p3 |
| 116 | Livermorium | *[Rn]5f146d107s27p4 |
| 117 | Tennessine | *[Rn]5f146d107s27p5 |
| 118 | Oganesson | *[Rn]5f146d107s27p6 |
FAQs
-
What are the 3 principles of electron configuration?
In order from lowest energy to highest energy, these three principles are Pauli Exclusion Principle, Aufbau principle, Hund’s Rule.
-
How do you write the electron configuration of an element?
The electron configuration of an element is the order in which electrons are arranged around the nucleus. To write the electron configuration of an element, you first need to know how many protons and neutrons are present in each atom.
Next, you must consider its position on the periodic table. If it’s a group 1 or group 2 element, then all of its electrons will be found at one outermost orbital level – this is called valence shell filling. However, if it’s a transition metal or metalloid (groups 3-12), then there will be multiple orbitals that contain electrons – these are called subshells. Electrons fill up subshells from lowest energy to highest energy until they’re completely filled before moving onto another subshell. -
What is the electronic configuration of 1 to 20 elements?
Elemental configuration is the arrangement of electrons in an atom’s energy level. This covers electronic configurations for 1 to 20 elements. There are some common trends that can be seen, such as how each successive element has one more electron than its predecessor.
-
How do you read electron configuration?
The electron configuration of an atom is the order in which electrons orbit around it. The first outermost shell has one electron, so its electron configuration would be 1s1. All subsequent shells are filled with two electrons each, so the 2nd shell’s electron configuration would be 1s2, and so on. The last shell is called the valence shell because it only contains electrons that can participate in chemical reactions.