Chapter 8: Chemical equilibrium
In this article, the author has explained the most important chemistry MCQs from the eighth chapter ( Chemical equilibrium ) of FSC chemistry. All the correct answers have been represented as bold.
Note: In this blog post following ↔ sign represents the reversible reaction
Related: FSC 1st year chemistry 1st chapter MCQs
Related: FSC 1st year chemistry 2nd chapter MCQs
Related: FSC 1st year chemistry 3rd chapter MCQs
1: Consider the following reaction in equilibrium with equilibrium concentration 0.01 M of every species
(I) PCl5 —-> PCL3 + Cl2
(II) 2HI —-> H2 + I2
(III) N2 + 3H2 —-> 2NH3
The extent of the reaction taking place is
a) I>II>III
b) I<II<III
c) II<III<I
d) III<I<II
2: Which of the following pairs could not be used for preparing a buffer solution?
a) CH3COOH —-> CH3COONa
b) H3PO4 + NaH2PO4
c) CaCl2 + Ca (OH)2
d) NaH2PO4 + NaOH
3: For the reaction 3 A(g) + B(g) à 2C (g) at a given temperature kc =9.0. What must be the volume of the flask, if a mixture of 2.0 mol each A, B, and C exist in equilibrium?
a) 6L
b) 9L
c) 36L
d) None of these
4: Very small value of Kc for a reaction at equilibrium indicates
a) Very small amount of reactants
b) Very small amount of products
c) The rate of backward reaction is greater than that of forward one
d) The rate of forward reaction is greater than that of backward one
Related: FSC 1st year chemistry 4th chapter MCQs
Related: FSC 1st year chemistry 5th chapter MCQs
Related: FSC 1st year chemistry 6th chapter MCQs
Related: FSC 1st year chemistry 7th chapter MCQs
Related: FSC 1st year chemistry 9th chapter MCQs-Solutions MCQs
Related: FSC 1st year chemistry 10th chapter MCQs-Electrochemistry Mcqs
5: Which of the following buffer is present in our blood plasma?
a) Acetic acid + Sodium acetate
b) Carbonic acid + Bicarbonates
c) Boric acid + Borax
d) Phthalic acid + Potassium acid phthalate
6: A solution X of pH = 2 has higher acidity than a solution Y pH = 6, the ratio of H+ of solution X to that of solution Y is
a) 10
b) 10000
c) 1000
d) 100000
7: For a reaction A + B ↔ 2C
2 moles of A and 3 moles of B are allowed to react if the equilibrium constant is at 4 at 400oc then the mole of C at equilibrium is
a) 1
b) 3.6
c) 2.4
d) 4
8: The conjugate acid of NH2- is
a) NH3
b) NH4+
c) NH2OH
d) N2H4
9: The pH of an aqueous solution is 1.0 M of a weak monoprotic acid which is 1% ionized is
a) 0
b) 2
c) 1
d) 11
10: The pH of 0.1 M solution of the following salts present in the order
a) NaCl < NH4Cl < NaCN < HCl
b) NaCN < NH4Cl < NaCl < HCl
c) HCl < NH4Cl < NaCl < NaCN
d) HCl < NaCl < NaCN < NH4Cl
11: Solubility product of PbCl2 at 298 K is 10-6. At this temperature solubility of PbCl2 in mol/L is
a) (10-6) ½
b) (0.25 X 10-6) 1/3
c) (10-6) 1/3
d) (0.25 X 10-6) ½
12: For reaction, H2 + I2 ↔ 2HI, the equilibrium constant Kp Changes with
a) Total pressure
b) catalyst
c) The amount of, H2 and I2 present
d) Temperature
13: The equilibrium constant for the reaction
PCl5 ↔ PCL3 + Cl2 is 16
If the original volume of the container is reduced to half of its original volume, the value of Kp for the reaction at the same temperature will be
a) 32
b) 16
c) 64
d) 4
14: In a reversible reaction at equilibrium, the concentration of reactants is
a) Always equal to that of products
b) Always smaller than that of products
c) Always greater than that of products
d) None of these
15: Which of the following is not true?
a) Kc = Kp/(RT)∆n
b) Kc = Kp(p)∆n
c) Sometimes Kc = Kp
d) All of these
16: Which of the following solutions would cause precipitation when added to a saturated solution of PbBr2?
a) NaNO3
b) Zn(NO3)2
c) AgCl
d) HBr
17: Which of the following represents a weak acid?
a) HNO3
b) H2S
c) CH3COONa
d) NH4OH
18: Which of the following can be used to produce a buffer of pH below?
a) NaCl + HCl
b) CH3COONa + CH3COOH
c) NH3 + NH4OH
d) NH4Cl + HCl
19: Solubility product of Ca(OH)2 is 8 x 10-6, Its solubility in water is approximately
a) 1 x 10-2 mol/dm3
b) 3 x 10-2 mol/dm3
c) x 10-2 mol/dm3
d) None of these
20: pH of 10-3 mol/dm3 aqueous solution H2SO4 is
a) 3.5
b) 1.9
c) 2.7
d) 0
21: In which of the following Kp is smaller than Kc?
a) N2O4 ↔ 2NO2
b) 2SO2 + O2 ↔ 2SO3
c) 2HI ↔ H2 + I2
d) N2 + O2 ↔ 2NO
22: The effect of increasing pressure on the system
2A + 3B ↔ 3C+ 2D indicates that
a) Forward reaction is favored
b) Backward reaction is favored
c) No effect is observed
d) It totally depends on other factors
23: Which of the following is an illustration of a reversible reaction?
a) Pb(NO3)2(aq) + 2Na(aq) —-> PBI2(s) + 2NaNO3(aq)
b) Ag(NO3)2(aq) + NaCl(aq) —-> AgCl(s) + NaNO3(aq)
c) 2NO(g) + 2H2(l) —-> 2NaNO3(aq) + H2(g)
d) KNO3(aq) + NaCl(aq) —-> KCl(aq) + NaNO3(aq)
24: The equilibrium constant for the reaction
2x(g) + Y(g) ↔ 2Z(g) is 2.25. What would be the concentration of Y at equilibrium with 2.0 moles of X and 3.0 moles of Z in one litre vessel?
a) 1.0 moles
b) 2.25 moles
c) 2.0 moles
d) 4.0 moles
25: In the mixture of NO and CO2 (initially containing 4 mol of NO and 0.9 mol of CO2) reaction occurs according to the equation below
NO(g) + CO2(g) ↔ NO2(g) + CO(g)
At equilibrium 0.1 mol of CO2 was present. What is the equilibrium constant Kc at the temperature of this experiment?
a) 0.2
b) 1.6
c) 0.5
d) 2.0
26: The pH of a 1.0 mol dm-3 solution of a weak monobasic acid is 4. What is the dissociation constant of the weak acid?
a) 1.0 x 10-2 moldm-3
b) 1.0 x 10-8 moldm-3
c) 1.0 x 10-7 moldm-3
d) 1.0 x 10 -7 moldm-3
27: The dissociation of dinitrogen tetraoxide into nitrogen dioxide is represented by the equation below.
N2O4(g)↔ 2NO(g) ∆H0 = + 57.2 kJ/mol
If the temperature of an equilibrium mixture of the gases is increased at constant pressure, the volume of the mixture will
a) Increase, but only because of a shift of equilibrium towards the right
b) Increase both because of a shift of equilibrium towards the right and also because of thermal expansion
c) Stay the same, because any thermal expansion could be exactly counteracted by a shift of equilibrium towards the left
d) Decrease, because a shift of equilibrium towards the left would more than counteract any thermal expansion
28: A sample of 1 mol of N2O4 was placed in any empty 1dm3 container and allowed to reach equilibrium according to the following equation.
N2O4(g) ↔ 2NO2(g)
At equilibrium, x mol of the N2O4 had dissociated. What is the value of the equilibrium constant Kc at the temperature of the experiment?
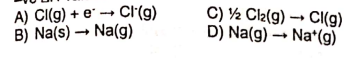
a) A
b) B
c) C
d) D
29: Which of the following affect the value of the solubility product Ksp of silver sulfide when it is precipitated by passing hydrogen sulfide into aqueous silver nitrate?
2AgNO3(aq) + H2S(g) ↔ Ag2S(s) + 2HNO3(aq)
a) An increase in temperature
b) The addition of aqueous silver nitrate
c) The addition of aqueous sodium sulfate
d) The pressure of the hydrogen sulfide
30: Ethanoic acid is a stronger acid in liquid ammonia than in water. The reason for this may be;
a) Ethanoic acid molecules form hydrogen bonds with water
b) Ethanoic acid is more soluble in liquid ammonia than in water
c) Ammonia is a stronger base than water
d) Ethanoic acid has a high enthalpy change of hydration
31: The pKb value for aqueous ammonia at 25oC is 4.8. What is the correct pKa value for the ammonium ion at this temperature?
a) -4.8
b) 4.8
c) 2.2
d) 9.2
32: Methanol is manufactured industrially by the catalytic reaction shown:
CO(g) + 2H2(g) —-> CH3OH(g) ∆H= -92 kJ/mol
The operating conditions are:
250oC; a pressure between 50 atm and 100 atm; A copper-based catalyst
Which factor influence the choice of these conditions?
a) The catalyst increase the equilibrium yield of methanol
b) At lower pressure, the rate of formation of methanol increases
c) At lower temperatures the equilibrium yield of methanol increases
d) At lower temperatures the rate of formation of methanol increases
33: In a reaction A2 +4B2 ↔ 2AB4; ∆H < O, The formation of AB4 will be favored by
a) Low temperature and high pressure
b) High temperature and low pressure
c) Low temperature and low pressure
d) High temperature and high pressure
34: Which of the following statements are true about the Haber process for the manufacture of
Ammonia?
a) At higher temperatures, the yield goes down but the rate of production of ammonia is faster
b) At higher pressures, the yield goes down but the rate of production of ammonia is faster.
c) In the presence of a catalyst, the yield goes up and the rate of production of ammonia is faster.
d) Both ‘a’ and ‘c’ are correct.
35: A reversible reaction is catalyzed, which of the following statements about this system are correct?
a) The catalyst alters the number of moles of products of the reaction.
b) Equilibrium position is shifted in the forward direction as a result of the increase in the rate.
c) The catalyst alters the composition of the equilibrium mixture.
d) None of these
36: Which of the following in aqueous solution does considerably change in pH when a relatively small volume of strong acid or strong alkali is added?
a) A mixture of carbonic acid and sodium hydrogen carbonate.
b) A mixture of sodium chloride and ethanoic acid
c) A mixture of sodium sulfate and sodium chloride.
d) None of these
37: The optimum industrial conditions for the synthesis of ammonia are
a) 100-200 atm. 4000C, Fe + Al203
b) 200-300 atm, 4000C, Fe embedded in Al203+ MgO + Si02
c) 200 atm, 100-2000C, V205.
d) 400 atm, 200-3000C, Fe embedded in Al203+ MgO + Si02
38: What is not correct for H2O at normal temperatures?
a) KW = 10-14
b) pOH = 7
c) [H+]= [OH–]
d) KW = [H+] [OH–]/[H20]
39: Which of the following statements is correct about a reaction for which the equilibrium constant is independent of temperature?
a) The activation energies for both forward and reverse reactions are zero
b) The enthalpy change is zero
c) Its rate constants do not vary with temperature.
d) There are equal numbers of moles of reactants and products.
40: The oxidation of S02 by 02 to S03 is an exothermic reaction. The yield of S03 will be maximum if
a) Temperature is increased and pressure is kept constant
b) Both temperature and pressure are increased
c) Temperature is reduced and pressure is increased
d) Both temperature and pressure are decreased
41: For the reaction
N2(g) + 3H2(g) 2NH3(g), ∆H = -93.6 KJ/mol the concentration of H2 at equilibrium can be increased by
(i) Lowering the temperature
(ii) Increasing the volume of the system
(iii) Adding N2 at constant volume
(iv) Adding H2 at constant volume
a) (ii) and (iv) are correct
b) (i), (ii) and (iii) are correct
c) Only (ii) is correct
d) (iii) and (iv) are correct
42: An acidic buffer solution can be prepared by mixing the solutions of
a) Sodium chloride and sodium hydroxide
b) Sulphuric acid and sodium sulfate
c) Ammonium chloride and ammonium hydroxide
d) Sodium acetate and acetic acid
43: The strongest Bronsted base among the following ions is
A) CH30–
B) (CH3)2CHO–
C) C2H50–
D) (CH3)3C0–
44: The compound whose 0.1 M solution is basic is
a) Ammonium acetate
b) Sodium acetate
c) Ammonium sulfate
d) Ammonium chloride
45: For a reversible reaction, if the concentrations of the reactants are doubled, at constant temperature the equilibrium constant will be
a) One-fourth
b) Halved
c) Doubled
d) The same
46: A reversible reaction is said to have attained equilibrium, when
a) Backward reaction stops
b) Both backward arid forward reactions stop
c) Both backward and forward reactions take place at equal speed.
d) Concentration of each of the reactants and products becomes equal
47: A vessel at equilibrium contains S03, S02, and 02. Now some helium gas is added, so that total
Pressure increases while temperature and volume remain constant. According to Le Chatelier’s
Principle, the dissociation of S03
a) Decreases
b) Remains unaltered
c) Increases
D) Change unpredictably
48: The chemical equilibrium of a reversible reaction is not influenced by
a) Temperature
b) catalyst
c) Pressure
d) Concentration
49: The pKa of acetylsalicylic acid (aspirin) is 3.5. The pH of gastric juice in the human stomach is about 2-3
and pH in the small intestine is 8. Aspirin will
a) Ionized in the small intestine and almost unionized in the stomach
b) Unionized in the small intestine and in the stomach
c) Completely ionized in the small intestine and in the stomach
d) Ionized in the stomach and almost unionized in the small intestine.
50: Which of the following favors the backward reaction in a chemical equilibrium?
a) Decreasing the concentration of one of the reactants
b) Increasing the concentration of one of the reactants
c) Increasing the concentration of one or more of the products
d) Removal of at least one of the products at a regular interval
51: Appropriate units of Kp for the following reaction is
2SO3(g) ↔ 2SO2(g) + O2(g)
a) mol/dm3
b) Torr
c) dm3/mol
d) dm6/mol2
52: At equilibrium, the relationship between concentrations of reactants and products
a) [Reactants] > (Products]
b) [Reactants] < [Products]
c) [Reactants) =
d) All are possible
53: If Ksp is equal to the product of the concentration of ions at a particular temperature then the solution is
a) Saturated
b) Supersaturated
c) Unsaturated
d) Concentrated
54: Which will change for the first-order reaction with time?
a) Rate constant
b) Rate of reaction
c) Half-life
d) All of these
55: When two reactants A and B are mixed to give products C and D the reaction quotient, Q at the initial stage of the reaction
a) is independent of time
b) is zero
c) Decrease with time
d) increase with time
56: Species acting both as Bronsted acid and base is
a) OH-
b) NH3
c) Na2CO3
d) HSO4–
57: At 90oC pure water has H3O+ ion concentration of 10-6 mol/L, the Kw at 90oC is
a) 10-8
b) 10-6
c) 10-14
d) 10-12
59: The pH of a solution obtained by mixing 50 ml of 0.4M HCl with 50 ml of 0.2 M NaOH is
a) –log2
b) 1.0
c) –log 2 x 10-1
d) 2.0
60: The solubility of A2X3 is y mol dm-3. Its solubility product is
a) 6y4
b) 36y5
c) 64y4
d) 108y5