Chapter 6: Chemical bonding
In this article, the author has explained the most important chemistry MCQs from the sixth chapter( Chemical bonding ) of FSC chemistry. All the correct answers have been represented as bold.
Related: FSC 1st year chemistry 1st chapter MCQs
Related: FSC 1st year chemistry 2nd chapter MCQs
Related: FSC 1st year chemistry 3rd chapter MCQs
1. The bond between two identical non-metal atoms has a pair of electrons:
A) With identical spins
B) Unequally shared the two.
C) Transferred fully from one atom to another
D) Equally shared between them
2. Which theory does give a satisfactory answer about the geometry of the molecules?
A) Valence bond theory (VBT).
B) Molecular Orbital Theory (MOT).
C) Valance shell electron pair repulsion (VSEPR) theory.
D) All of these
3. Ionic compounds do not show the phenomenon of isomerism because the ionic bonds are
A) Directional and rigid.
B) Non-directional and non-rigid.
C) Non-directional and rigid
D) The entire above are true.
4. The correct Order Of energy for molecules is;
A) Cl2 > Br2 > F2 > I2
B) I2> Br2 > Cl2> F2
C) F2> Cl2> Br2> I2
D) Cl2 > I2> Br2 > F2
5. Which of the following orders is true for boiling points?
A) NaCl>CH3CH20H>NH3>CH3OCH3
B) NaCl>CH3CH20H> CH3OCH3>NH3
C) NaCl>CH3CH20H>NH3>CH3OCH3
D) NaCl>CH3CH20H> CH3OCH3>NH3
6. Which one of the following is a linear molecule?
A) H20
B) Cl20
C) HCN
D) C2H4
7. In which one of the following does the underlined atom not has eight electrons in its valence shell?
A) Li2O (O=8)
B) Na2O (Na=11)
C) H2S (S=16)
D) PCl5 (P=15)
Related: FSC 1st year chemistry 4th chapter MCQs
Related: FSC 1st year chemistry 5th chapter MCQs
Related: FSC 1st year chemistry 7th chapter MCQs
8. The reason for the planar geometry of BCI3 and non-planar one for PH3 is
A) B atom has no d-orbitals for bonding
B) B atom has six electrons around it in BCl3 where P in PH3 has eight.
C) The repulsion between chlorine atoms is greater than that between H atoms
D) The covalent radius Of P is greater than that of B
Related: FSC 1st year chemistry 8th chapter MCQs-chemical equilibrium MCQs
Related: FSC 1st year chemistry 9th chapter MCQs-Solutions MCQs
Related: FSC 1st year chemistry 10th chapter MCQs-Electrochemistry Mcqs
9. Which of the following statements about the properties associated with ionic and covalent is correct?
A) A covalent compound can’t be an electrolyte.
B) The only covalent compounds with high melting points are those in which hydrogen bonds occur.
C) Any compound that contains both oxygen and hydrogen in its molecule forms a hydrogen bond
D) Ionic bonds and covalent bonds can both occur in the same compound.
10. The approximate value of the O-C-O angle in ethanoic acid is
A) 90o
B) 109o
C) 120o
D) 180o
11. Graphite used as a lubricant, diamond can’t. This is because graphite has
A) Delocalised electrons.
B) A hexagonal arrangement of atoms.
C) Covalent atoms between atoms in the layers.
D) Van der Waals’ forces between the layers of atoms
12. Which one of the following pairs do they have similar shapes, in?
A) AICl3 and BCl3
B) BF3 and NH3
C) AICl3 and PCl3
D) BeCl2 and H2O
13. A solid melts sharply just above I00oC. It does not conduct electricity even when molten, It dissolves in hydrocarbons solvents. What is the structure of solid most likely to be?
A) An atomic crystal
B) A giant molecular crystal
C) An ionic crystal
D) A molecular crystal
14. Which of the following molecules will not form a hydrogen bond with another of its own molecules?
A) CH3CHO
B) CH30H
C) CH3NH2
D) NH3
15. Liquid hydrocarbons float on the surface of the water. Which of the following statements helps to explain why liquid hydrocarbons both float on and are less dense than water?
l. There are only Van der Waals’ interactions between hydrocarbon molecules.
II. Hydrogen bonding between the molecules in liquid water causes them to pack close together.
Ill. Hydrocarbon molecules are not solvated by water
IV. Hydrocarbons are organic while water is not
A) Only I
B) Both I and II
C) I, II, and III
d) All of these
16. Group II metals have higher melting points than Group I metals. Which factor could contribute towards the higher melting points?
A) There are smaller inter-atomic distances in the metallic lattices of the Group II metals.
B) Two valence electrons are available from each Group II metal atom for bonding the atom into the metallic lattice
C) Group II metals have the higher first ionization energies.
D) None of the above
17. Which Of the following Of aluminum chloride are related to the lack of an octet Of electrons in the aluminum atom in this compound
A) Its tendency to dimerize.
B) Its acidity in an aqueous solution.
C) Its covalent character.
D) Its reaction with bases.
18. Which of the following molecules has polar bonds but is non-polar as a whole?
A) HCl
B) CO2
C) NH3
D) H2O
19. Among the following species, identify the isostructural pairs, NF3, NO3-, BF3_, H3O+, HN3
A) [NF3, NO3–] and [BF3_, H3O+]
B) [NF3, H3O+] and [NO3–, BF3_]
C) [NF3_, HN3] and [NO3–, BF3_]
D) [NF3_, H3O+] and [HN3, BF3_]
20. In which of the following the central atom does not use sp3 hybrid orbitals in its bonding
A) NF3
B) H3O+
C) NH2–
D) BeF3–
21. Which of the following characteristics apply to PCl3?
1. Non-polar molecule
2. Polar bonds
3. Trigonal-pyramidal molecular geometry,
4. sp2 hybridized
A) I and 2
B) 3and4
C) 2 and 3
D) 1, 2, and 3
22. Bonds present in CuSO4.5H2O is
A) Electrovalent and covalent bonds
B) Electrovalent and coordinate bonds
C) Covalent and coordinate bonds
D) Electrovalent, covalent and coordinate covalent bonds
23: Which of the following molecules or ions is nonpolar, i.e., has at least one atom is not in the same plane as the others?
a) BH3
b) NF3
c) SO3
d) CO32-
24: The shape of hydronium ion is H3O, is
a) Planar
b) See-saw
c) trigonal planar
d) trigonal pyramidal
25: the type of bonding present in a sample of sodium nitrate, NaNO3 are;
a) Covalent bonds only
b) Ionic bond only
c) Ionic and metallic bonds
d) Covalent and ionic bonds and coordinate covalent
26: What is the hybridization of phosphorus in PCl3?
a) Sp2
b) Sp3
c) sp
d) sp3d2
27: A molecule with the formula AB2 uses ——- to form its bonds?
a) sp hybrid orbitals
b) sp3 hybrid orbitals
c) sp2 hybrid orbitals
d) sp3d hybrid orbitals
28: Which one of the following statements about orbital hybridization is incorrect?
a) the nitrogen atom in NH3 is sp3 hybridized
b) sp2 hybrid orbitals are coplanar and at 120o to each other
c) the carbon atom in CO2 is sp2 hybridized
d) sp hybrid orbitals lie at 180o to each other
29: Which of the following is the correct order of bond angles?
a) NH3>NH4+>NH2–
b) NH4+ > NH3 > NH2–
c) NH2 > NH4+ > NH3
d) NH3 > NH4+ > NH2–
30: The ratio of sigma and pi bonds in benzene is
a) 1:2
b) 1:6
c) 1:4
d) 1:6
31: in 1,3-butadiene the carbon is hybridized as
a) sp
b) sp2 and sp3
c) sp3
d) sp2
32: The molecule having the highest bond energy is
a) N-N
b) F-F
c) C-C
d) O-O
33: Which one of the following is the correct set with reference to molecular formula, hybridization of the central atom, and shape of the molecule?
a) CO2, sp2, bent
b) BeCl2, sp, linear
c) H2O, sp2, bent
d) H2O, sp3, linear
34: In all of the following molecules the central atom does not follow the octet rule except?
a) PCl5
b) H2SO4
c) BF3
d) None of these
35: In which of the following species, intramolecular H-bonding occurs?
1: acetate ion 2: Salicylate ion
3: Propanoic acid 4: O-nitrophenol
a) 1,3,4
b) 2,4
c) 1,2,4
d) 4 only
36: H2O is a polar molecule whereas BeF2 is not. It is because
a) The electronegativity of F is greater than that of O
b) H2O involves hydrogen bonding whereas BeF2 is a discrete molecule
c) H2O is angular and B3F2 is linear
d) H2O is linear and BeF2 is angular
37: Which type of bond is not present in the HNO2 molecule?
a) Covalent
b) ionic
c) Coordinate
d) Ionic as well as coordinate
38: Which of the following is true?
a) Bond energy ∞ 1/bond length ∞ Bond order
b) Bond energy ∞ Bond order ∞ Bond length
c) Bond energy ∞ 1/bond length ∞ 1/Bond order
d) Bond order ∞ Bond length ∞ bond energy
39: Which of the following statements about the bond is wrong?
a) Polar bonds are shorter than the non-polar ones
b) C-C bond length is smaller than S-S bond length
c) pi-bonding reduces the bond length
d) A single bond is shorter than a double bond
40: the correct order of increasing C-O bond length of CO, CO32–, CO2 is
a) CO32- < CO2 < CO
b) CO < CO32- < CO2
c) CO2 < CO32- < CO
d) CO < CO2 < CO32-
41: The formation of macromolecules is characteristics of
a) covalent bond
b) ionic bond
c) Coordinate covalent bond
d) All of these
42: Predict the relative bond angles in BF3 and So2
a) BF3 bond angles > So2 bond angle
b) SO2 bond angle > BF3 bond angles
c) BF3 bond angles = SO2 bond angle
d) Relative bond angles cannot be predicted
43: Isostructural species are those which have the same shape and hybridization. Among the given species identity the isostructural pairs
a) NF3 and NH4+
b) BF4– and NH4+
c) BCL3 and BeCl3
d) NH3 and NO3–
44: In NO3– ion the number of bond pairs and lone pairs of electrons on nitrogen atom are
a) 2, 2
b) 1, 3
c) 3, 1
d) 4, 0
45: The dipole moments of NH3, HF, H2O, and So2 are 1.44 D, 1.9 D, 1.84D, and 1.60 D respectively. The order of decreasing polarity is
a) HF > SO2 > H2O > NH3
b_ H2O > NH3 > So2 > HF
c) HF > NH3 > So2 > H2O
d) Hf > H2O > SO2 > NH3
46: Which set of approximate bond angles at C1, C2, and N of the following molecule indicates the correct shape?
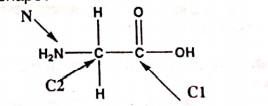
a) C1 120o, C2 120o, N 120o
b) C1 109o, C2 120o, N 109.5o
c) C1 109.5o, C2 120o, N 120o
d) C1 120o, C2 109.5o, N 109.5o
47: What hybridization change does the carbon atom undergo in the combustion of methane?
a) sp –> sp2
b) sp3 –> sp
c) sp2–> sp3
d) sp2 –> sp
48: Which of the following orbital shows the shortest bond length on overlapping?
a) sp
b) sp2
c) sp3
d) P
49: Bond responsible for dipole moment is
a) ionic
b) covalent
c) coordinate covalent
d) Metallic
50: Total number of sigma and pi bonds in 1,3-butadiene respectively are
a) 9,3
b) 9,2
c) 8,2
d) 4, 2
51: Bond created by overlapping of one modified orbit on another orbit is known as
a) Sigma bond
b) Pi bond
c) Covalent bond
d) Dative bond
52: which type of bonding would be expected between S and Cl?
a) ionic
b) non-polar covalent
c) Polyionic
d) polar covalent
53: Which formula represents a molecular substance?
a) caO
b) Co
c) Li2O
d) Al2O3
54: How are bond length and bond energies related?
a) they are not related
b) The lower the bond energy, the shorter the bond length
c) The higher the bond energy, the longer the bond length
d) The higher the bond energy, the shorter the bond length
55: Which kinds of bonding can be found in a sample of H2O (I)?
a) both polar covalent and hydrogen bonds
b) hydrogen bonds only
c) ionic and nonpolar hydrogen bonds
d) Nonpolar covalent bonds only
56: Carbon tetrachloride has no net dipole moment because of
a) Similar electron affinities of carbon and chlorine
b) Its planar structure
c) similar sizes of carbon and chlorine atoms
d) Its regular tetrahedral structure
57: Which sequence of group 18 elements demonstrate a gradual decrease in the strength of the Van der Waals forces? All the choices are elements in the liquid state:
a) Ar, Kr, Ne, Xe
b) Kr, Xe, Ar, Ne
c) Xe, Kr, Ar, Ne
d) Ne, Ar, Kr, Xe
58: On hybridization of one s and one p orbitals we get
a) Two mutually perpendicular orbitals
b) Two orbital at 180o
c) Three orbital in a plane
d) four orbitals directed tetrahedrally
59: Which substance is an example of a network soild?
a) Nitrogen dioxide
b) Sulfur dioxide
c) Carbon dioxide
d) Silicon dioxide
60: Which bond has the greatest ionic character?
a) H—Cl
b) H—N
c) H—F
d) H—O
61: The number of lone electron pairs in the Ne molecule is
a) 1
b) 2
c) 4
d) 3
62: Which of the following covalent bonds has the greatest polarity?
a) S-O
b) Br-Br
c) C-P
d) B-O
63: Which compound contains no ionic bond?
a) CaO
b) K2O
c) NH4Cl
d) CO
64: The forces of attraction that exist between nonpolar molecules are called
a) Van der Walls/ dispersion forces
b) Ionic forces
c) Electrovalent bonds
d) Covalent bonds
65: The electron dot formula for AsCl3 shows:
a) Two single bonds, one double bond, and 9 lone pairs
b) One single bond, two double bonds, and 8 lone pairs
c) A total of 84 electrons dots
d) Three single bonds and 10 lone-pairs
66: Which of the following occurs in an ionic bond?
a) Like-charged ions attract
b) Two atoms share more than two electrons
c) Oppositely charged ions attract
d) Two atoms share two electrons
67: The model for metallic bonding is known as—–
a) The Rutherford model
b) The quantum model
c) The electron sea model
d) The electron cloud model
68: When H+ forms a bond with H2O to form the hydronium ion H3O+, this bond is called a coordinate covalent bond because…
a) It forms an especially strong bond
b) The electrons are equally shared
c) both bonding electrons come from the oxygen atom
d) The oxygen no longer has eight valence electrons
69: The less the electronegativity difference between two bonded atoms, the greater the…..
a) Metallic character
b) Ionic character
c) Covalent character
d) Polar character
70: the melting points of molecular compounds are usually……
a) Lower than those of ionic compounds
b) Below zero
c) Equal to those of ionic compounds
d) Higher than those of ionic compounds