Learning objectives
In this article, the author has explained How is distribution law modified by change in Molecular state
Relation between distribution law and change in molecular state
Table of Contents
It was pointed out by Nernst that C1/C2 is constant only if the solute exists as simple molecules in the two solvents.
If the solute undergoes association or dissociation in one of the solvents, it is found that C1/C2 is not constant.
In these cases distribution law applies only to that part of the solute which is present as simple molecules.
1. When solute undergoes association
Suppose the solute is present as simple molecule X in solvent A.
In solvent B n molecules of X associate to form Xn molecules.
Assuming that a few single molecules X are also present in solvent B. The equilibrium that exist in the two solvents are shown in the image below.
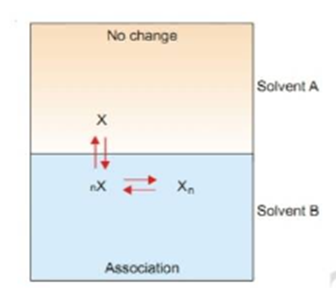
Let C1 be concentration of X in solvent A.
C3 be concentration of X in solvent B.
C2 be concentration of Xn in solvent B.
Applying distribution law to the equilibrium
X in solvent ⇌ X in solvent B
We have C1/C3=Kd —1
Applying mass law to the chemical equilibrium
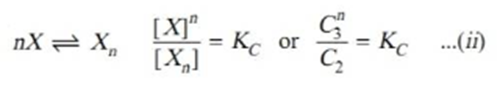
Taking nth root on both sides in equation ii.
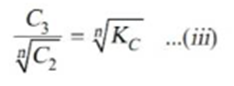
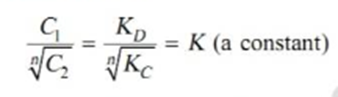
Thus when association occurs in one solvent the distribution equation is modified as following:
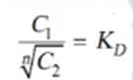
Since the solute exists largely as associated molecules, the total concentration of X determined experimentally in solvent B is taken as the concentration of the associated molecules Xn.
Solved Problem:
When Benzoic acid was shaken with mixtures of benzene and water at constant temperature the following results were obtained:
| Concentration of acid in benzene C1 | 0.24 | 0.55 | 0.93 |
| Concentration of acid in water C2 | 0.015 | 0.022 | 0.029 |
Solution
Calculating the ratio C1/C2 for each case.
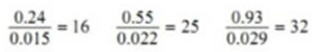
Thus the distribution coefficient is not constant. Therefore benzoic acid does not exist as single molecules in both solvents.
On calculating
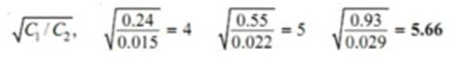
The constant value of partition coefficient now arrived at suggests that benzoic acid is associated into double molecules in the benzene layer.
2. When solute undergoes dissociation
Suppose the solute is present as normal molecules X in solvent A and it dissociates into A + B. The equilibrium set up in the two solvents are shown in image below.
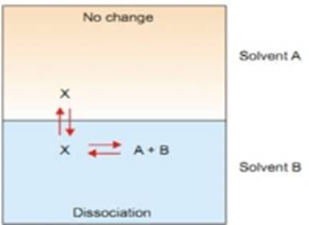
Let C1 be the concentration of X in solvent A and C2 the total concentration of X either dissociated and dissociated in solvent B. If the degree of dissociation in solvent B is X, then following equations makes the idea clear.

Hence the concentration of the undissociated or normal molecules in solvent B is C2(1-x). Applying distribution law to normal molecules in the two solvents:
C1/C2(1-x)=Kd
This is the modified distribution law equation when there is dissociation in one of the solvents.
- A case of this type arises in the distribution of a weak acid (such as succinic acid or oxalic acid) between ether and water.
- C1 and C2 can be determined by direct titration of the two layers against standard alkali solution.
- The value of x can be found by measuring electrical conductance of solution in solvent B.