Written by Adeel Abbas
Chromatography is a technique used to separate a mixture of chemical compounds into its components.
Definition of chromatography
Imagine you have a jar of mixed colored candies, red, blue and green. And you want to separate the candies based on their colors. One way you could do this is by taking a piece of white filter paper, dipping one end into the jar, and then holding the other end. The candy mixture will begin to move up the paper as the liquid (mobile phase) evaporates.
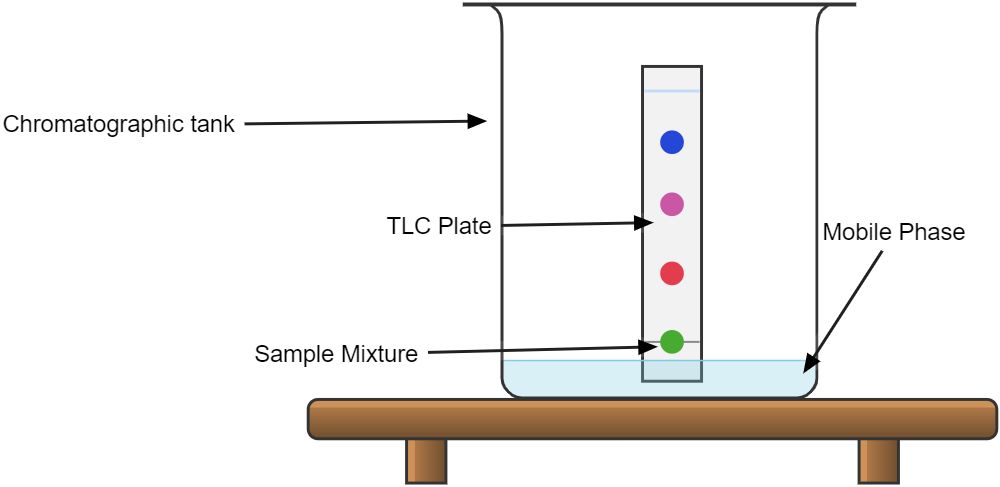
As the mixture moves, the different candies will stick to the paper at different rates, based on how much they dissolve in the liquid. So the red candies will move slower than the blue and green. After some time, the candies will be separated on the paper, based on their colors.
This is similar to how chromatography works, but instead of filter paper, scientists use different types of stationary phases and mobile phases, depending on the type of mixture they are trying to separate.
For example, in liquid chromatography, the stationary phase is a special type of column filled with beads, and the mobile phase is a liquid that flows through the column. And in gas chromatography, the stationary phase is a special type of coating inside a column and the mobile phase is a gas. The separated components can be collected and analyzed separately.
This process like other experimental techniques in chemistry is also widely used in industries, especially in the pharmaceutical and chemical field, to separate and purify compounds or to identify unknown components in a mixture.
History of chromatography
Table of Contents
Chromatography is a technique used to separate and analyze mixtures of chemical compounds. The history of chromatography can be traced back to the early 20th century, when the Russian botanist Mikhail Tsvet (also spelled “Tswett”) first used the technique to separate plant pigments. In 1901, Tsvet published a paper in which he described the use of a technique he called “chromatography,” from the Greek words for “color” and “writing,” to separate pigments from leaves and flowers.
After Tsvet, several other scientists made further developments in chromatography. For example, Archer Martin and Richard Synge developed partition chromatography in the 1940s, and in the 1950s, James and Martin developed gas chromatography. In the 1960s and 1970s, new types of chromatography such as high-performance liquid chromatography (HPLC) and affinity chromatography were developed.
Nowadays, Chromatography is a well-established technique, and it’s widely used in chemistry and biochemistry, as well as in fields like pharmacology, environmental science, and food science. It continues to evolve and improve to tackle even more complex separations and analysis.
Important terms in chromatography
There are many terms and concepts used in chromatography, here are a few common ones:
Column: The cylindrical piece of equipment that holds the stationary phase and through which the mobile phase flows in chromatography.
Mobile phase: The liquid or gas that flows through the column in chromatography and carries the sample through the stationary phase.
Stationary phase: The material that is packed into the column and does not move during chromatography. It is typically a solid or a gel, and it is chosen to interact selectively with the components of the sample.
Retention time: The amount of time it takes for a component of the sample to pass through the column and emerge from the other end. The retention time is affected by the properties of the stationary phase, the mobile phase, and the sample components themselves.
Resolution: The ability of the chromatography technique to distinguish between two or more components in a sample. It can be measured by the distance between two peaks in a chromatogram, relative to their widths.
Peak: A distinct region in a chromatogram that represents a component of the sample. It will typically have a maximum, also called a peak apex that indicates the time of maximum concentration of the component in the mobile phase
Sample injection: A way to introduce a small volume of the sample into the mobile phase at the start of the chromatography.
Chromatogram: A graph that shows how the components of a sample are separated by the chromatography.
Elution: The process of removing a component from the column after it has been separated.
Mobile phases and stationary phases for different types of chromatography
| Chromatography Type | Mobile Phase | Stationary Phase |
| Column Chromatography | Hexane, ethyl acetate, chloroform | Silica, alumina, activated charcoal |
| Gas Chromatography | Helium, nitrogen, hydrogen | DB-5, DB-17, Carbowax |
| Thin-layer Chromatography | Ethyl acetate, hexane, acetone | Silica gel, alumina |
| High-performance liquid chromatography (HPLC) | Water, methanol, acetonitrile | C18, C8, phenyl |
Principles of Chromatography
Chromatography is based on the principle of differential partitioning of the components of a mixture between a stationary phase and a mobile phase. The stationary phase is a solid or liquid material that is immobilized in a column or on a solid support, while the mobile phase is a liquid or gas that is passed through the column or over the solid support. The components of the mixture are separated based on their different affinities for the stationary and mobile phases.
Types of Chromatography
There are two main types of chromatography: adsorption chromatography and partition chromatography. In adsorption chromatography, the components of the mixture are separated based on their different affinities for the stationary phase. In partition chromatography, the components of the mixture are separated based on their different affinities for the mobile phase.
Some of the most commonly used chromatography methods include:
Column Chromatography
Column Chromatography is a method in which a mixture is passed through a column packed with a stationary phase. The column can be made of glass, metal, or plastic, and the stationary phase can be a solid or a liquid.
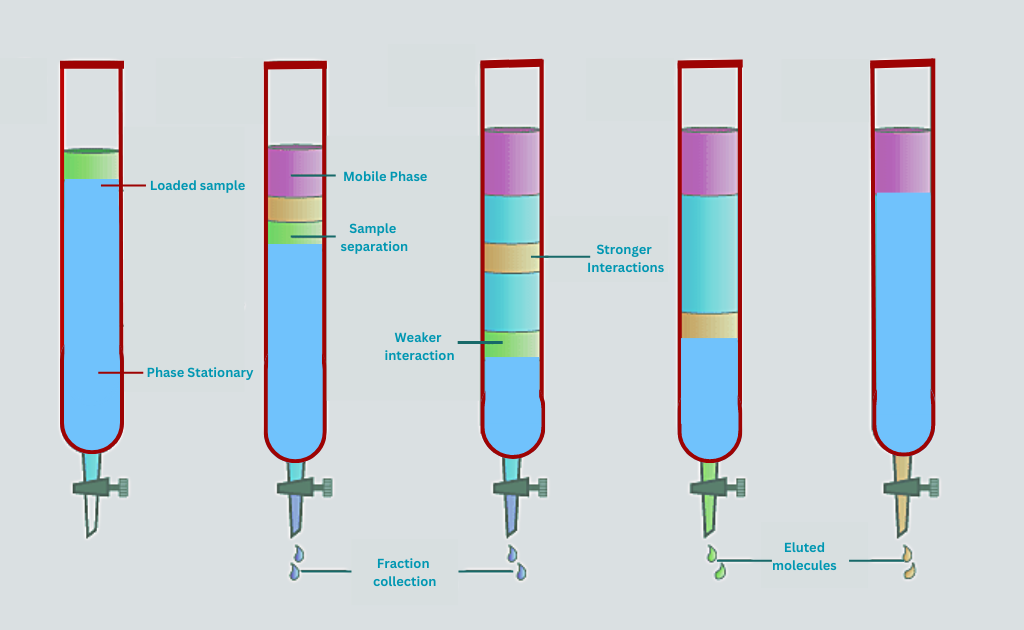
There are several different types of chromatography that are based on different principles and use different types of stationary and mobile phases. Here is a list of some common types of chromatography:
Gas chromatography (GC)
A type of chromatography in which the mobile phase is a gas and the stationary phase is a liquid or a solid that is coated onto a solid support. It is commonly used to separate and analyze volatile compounds, such as those found in gases and liquids.
Liquid chromatography (LC)
A type of chromatography in which the mobile phase is a liquid and the stationary phase is a solid, such as beads packed inside a column. It can be further divided into subtypes such as:
Thin-layer chromatography (TLC)
A type of chromatography in which the stationary phase is a thin layer of solid material, such as silica gel or alumina, that is coated onto a plate. The mobile phase is a liquid that is applied to the plate and then allowed to move up the plate by capillary action.
Paper chromatography
A type of chromatography similar to TLC but uses a filter paper as stationary phase and a liquid as mobile phase.
Gel filtration chromatography
A type of chromatography that separates molecules based on their size. It is also known as size exclusion chromatography, the stationary phase is beads with different sizes pore and the mobile phase is liquid.
Ion exchange chromatography
A type of chromatography that separates molecules based on their charge, it uses beads packed in a column as a stationary phase and a liquid as mobile phase.
Affinity chromatography
A type of chromatography that separates molecules based on their specific interactions, such as protein-ligand interactions. It uses a stationary phase that is immobilized with a molecule that has a specific interaction with the target compound in the mixture.
Supercritical fluid chromatography (SFC)
A type of chromatography in which the mobile phase is a supercritical fluid, such as carbon dioxide, and the stationary phase is a solid support.
Uses of chromatography
Chromatography is a widely used technique with many different applications. Here are a few examples of how it is used:
Analyzing and purifying pharmaceuticals
Chromatography is widely used in the pharmaceutical industry to purify drugs and analyze the components of drug compounds.
Analyzing food and drink
Chromatography is also used in food science and technology to analyze the composition of food and drink products, such as detecting and measuring additives, contaminants, and other substances.
Analyzing environmental samples
Chromatography is used in environmental science to analyze and monitor pollution in air, water and soil.
Identifying and quantifying chemicals in forensic analysis
Chromatography is used in forensic science to analyze and identify unknown substances, such as those found at crime scenes.
Biochemistry and biomolecules analysis
Chromatography is used in the fields of biochemistry, molecular biology, and genetics to purify and analyze biomolecules such as proteins, enzymes, DNA, and RNA.
Industrial analysis
Chromatography is widely used in the chemical and petroleum industries to analyze and purify compounds in raw materials and finished products.
Medical diagnostics
Chromatography is used in medical diagnostics to measure and analyze various bodily fluids such as blood, urine and saliva, to detect diseases, monitor treatment, and follow up on patient’s conditions.
Aerospace and defense
Chromatography is used to analyze and purify propellants and other chemicals used in aerospace and defense applications, ensuring their stability and safety.
Chromatography is a powerful technique that allows us to separate and analyze complex mixtures of chemical compounds. By understanding the basic principles of chromatography and the different types of chromatography methods available, scientists can select the most appropriate method for their specific research needs. It has wide application in Industries like food, biotechnology, forensic, environment, pharmaceuticals etc. It has become an indispensable technique in the field of analytical chemistry.
Read All chromatography related articles
- Thin Layer Chromatography (TLC): An Introduction
- Ascending and Descending Chromatography: A Brief Overview
- Low-Pressure Liquid Chromatography (LPLC)-A detailed guide
- High-Performance Liquid Chromatography (HPLC)- A detailed guide
- 10 Examples of Mixtures that Can be Separated by Chromatography
- Column Chromatography-Principle, Types, Applications
- Gas chromatography-intro, principle, working, applications
- Chromatography-Principle, types, uses and History