Written By Adeel Abbas
What is Thin Layer Chromatography?
Table of Contents
Thin layer chromatography (TLC) is a widely used separation technique in analytical chemistry that is used to separate and identify different compounds present in a mixture. TLC is a quick and easy-to-use method that is used for qualitative and quantitative analysis of a wide range of samples. In this article, we will discuss the basics of TLC, including its principle, technique, and applications.
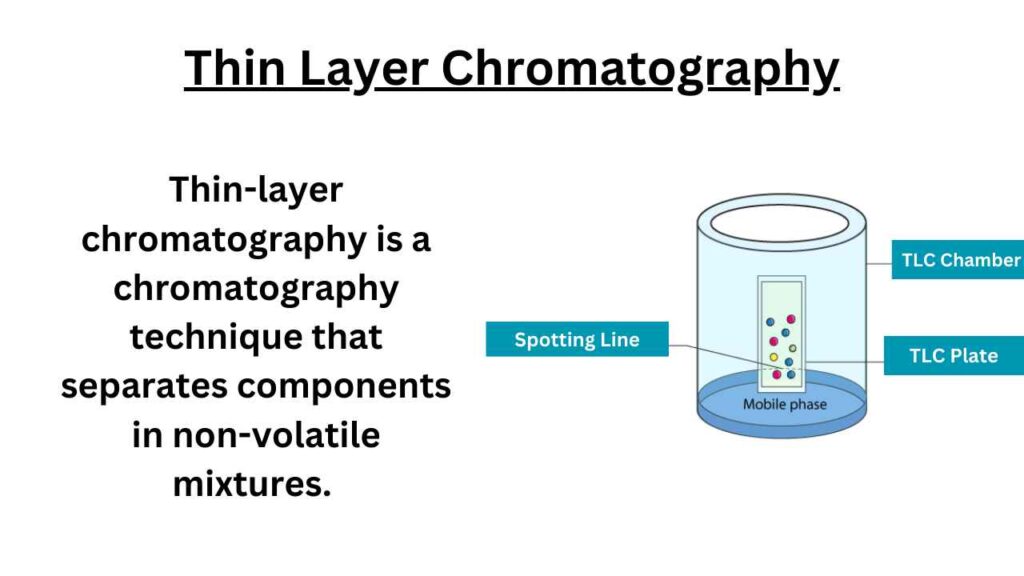
Principle of Thin Layer Chromatography
The principle of TLC is based on the separation of a mixture of compounds into individual components on a thin layer of adsorbent material (usually silica gel or alumina) supported on a flat plate.
The sample mixture is spotted at the bottom of the plate, and the plate is then placed vertically in a chamber containing a suitable solvent (the mobile phase).
The solvent then moves up the plate by capillary action, carrying the individual components of the mixture with it.
The components are separated on the plate based on their different interactions with the stationary phase (the adsorbent material) and the mobile phase (the solvent). The separated components can then be visualized using various techniques such as UV light or by using a staining reagent.
Process of Thin Layer Chromatography
TLC involves several steps, including the selection of stationary and mobile phases, sample preparation, and spotting.
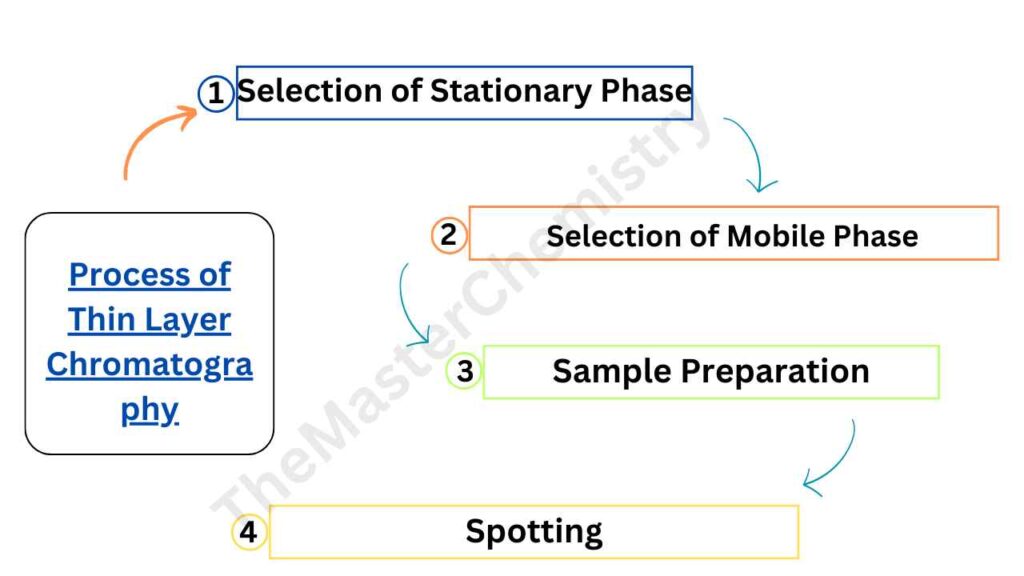
1: Selection of Stationary Phase
The stationary phase is a thin layer of adsorbent material (such as silica gel or alumina) that is coated onto a flat plate (usually glass). The selection of the stationary phase is based on the type of compounds that need to be separated. Silica gel is commonly used for separating nonpolar compounds, while alumina is used for polar compounds.
2: Selection of Mobile Phase
The mobile phase is the solvent that is used to move the components of the sample mixture up the plate. The selection of the mobile phase is based on the polarity of the components. A polar solvent is used for separating nonpolar components, while a nonpolar solvent is used for polar components.
3: Sample Preparation
The sample needs to be prepared before it is spotted on the plate. The sample can be dissolved in a suitable solvent or mixed with a suitable reagent to make it visible.
4: Spotting
The sample is spotted at the bottom of the plate using a capillary tube or a micropipette. The sample should be spotted as a small, concentrated spot to ensure good separation.
Interpretation of Results
After the solvent has moved up the plate and separated the components, the plate needs to be visualized to interpret the results. The separated components can be visualized using UV light or by using a staining reagent. The Rf value (retardation factor) of each component is calculated to identify the components.
Applications of Thin Layer Chromatography
TLC is used in a wide range of applications, including the following:
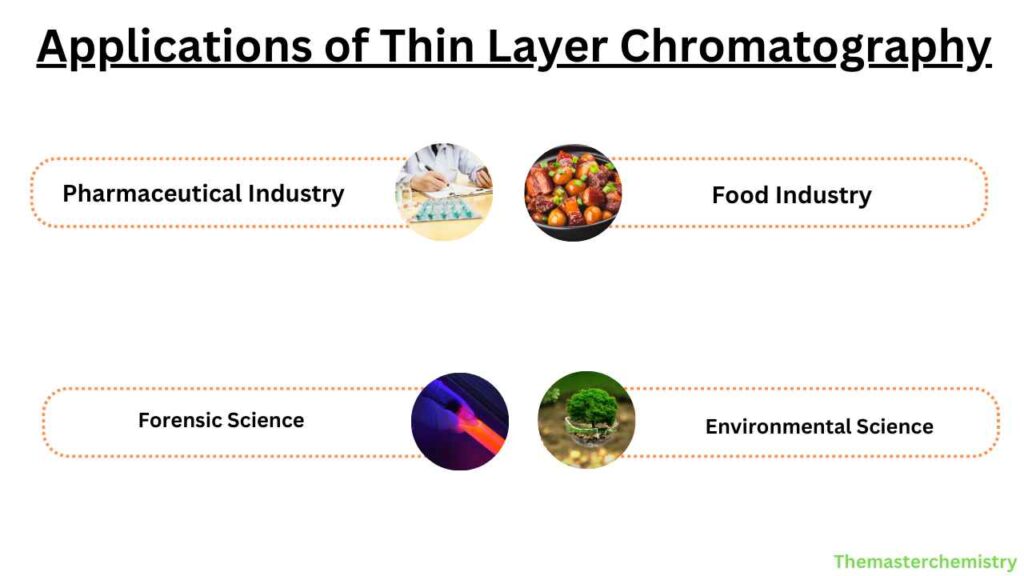
Pharmaceutical Industry
TLC is used in the pharmaceutical industry for the analysis of raw materials, intermediates, and final products. TLC is used for the identification and quantification of active ingredients, impurities, and degradation products.
Food Industry
TLC is used in the food industry for the analysis of food additives.
Forensic Science
TLC is used in forensic science for the analysis of trace evidence such as drugs, gunshot residues, and explosive residues. TLC is a useful tool for identifying unknown substances found at a crime scene.
Environmental Science
TLC is used in environmental science for the analysis of pollutants in soil, water, and air samples. TLC can be used to detect and quantify the presence of pollutants such as pesticides, herbicides, and PCBs in environmental samples.
Advantages and Disadvantages of Thin Layer Chromatography
TLC has several advantages and disadvantages that need to be considered before using this technique.
Advantages
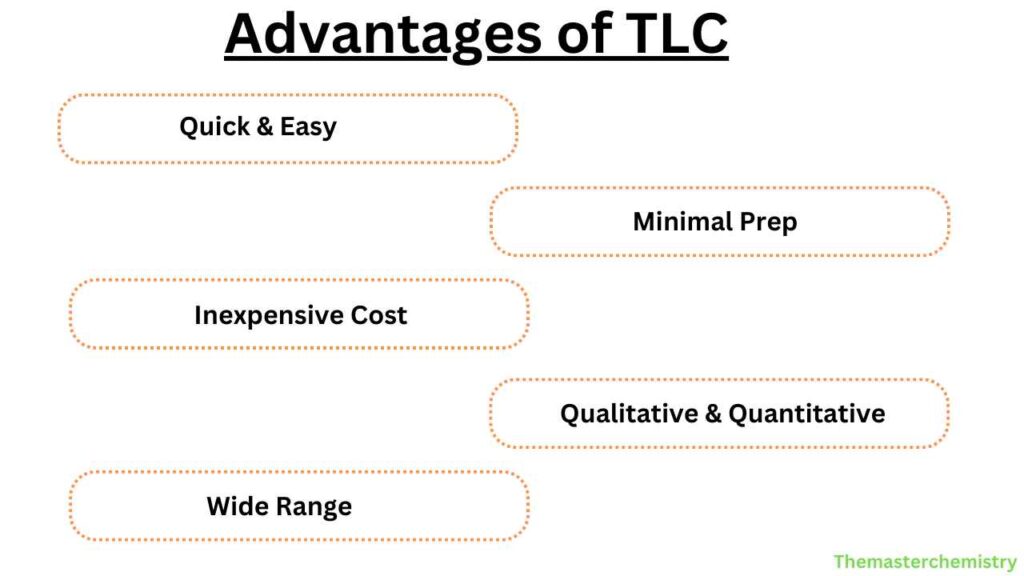
- Quick and easy to perform
- Requires minimal sample preparation
- Inexpensive compared to other chromatography techniques
- Can be used for both qualitative and quantitative analysis
- Can separate a wide range of compounds
Disadvantages
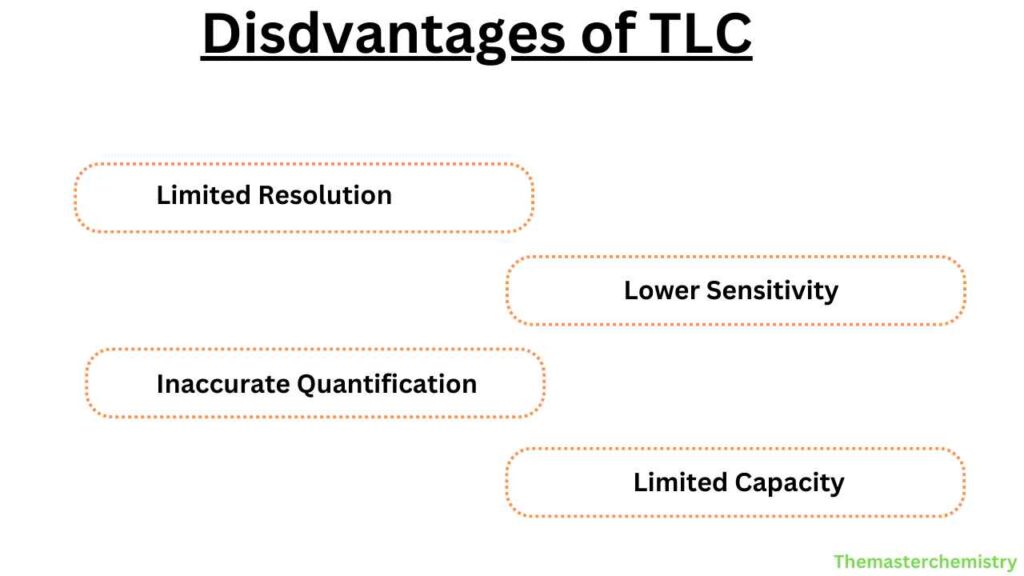
- Limited resolution compared to other chromatography techniques
- Sensitivity is not as high as other chromatography techniques
- Difficult to quantify the separated components accurately
- Sample capacity is limited
FAQs
Q1. What is the difference between TLC and HPLC?
A: TLC is a quick and easy-to-use method that is used for qualitative and quantitative analysis of a wide range of samples. HPLC (High-Performance Liquid Chromatography), on the other hand, is a more sophisticated technique that is used for the separation and quantification of compounds in complex mixtures.
Q2. What is the importance of the Rf value in TLC?
A: The Rf value (retardation factor) is a measure of the distance traveled by a component relative to the distance traveled by the solvent front. The Rf value is used to identify the components of a mixture and to compare the results of different TLC runs.
Q3. What is the stationary phase in TLC?
A: The stationary phase is a thin layer of adsorbent material (such as silica gel or alumina) that is coated onto a flat plate. The selection of the stationary phase is based on the type of compounds that need to be separated.
Q4. Can TLC be used for the analysis of volatile compounds?
A: No, TLC is not suitable for the analysis of volatile compounds. Gas chromatography (GC) is a more suitable technique for the analysis of volatile compounds.
Q5. What are the advantages of TLC over other chromatography techniques?
A: TLC is quick and easy to perform, requires minimal sample preparation, and is inexpensive compared to other chromatography techniques. TLC can be used for both qualitative and quantitative analysis and can separate a wide range of compounds.