Written by Adeel Abbas
Experimental techniques in the chemistry
Table of Contents
Chemistry is the science of experiments. Researchers try to answer questions with the help of experiments. Therefore, the role of experimental techniques is worth importance.
Experimental techniques in chemistry offer a lot of services to researchers, for example, purification, isolation, quality enhancement, etc. Importance of experimental techniques is increased in food and pharmaceutical industry. Because both these industries are related to the human survival.
There are many experimental techniques in chemistry such as…
Filtration, crystallization, sublimation, solvent extraction, chromatography, etc.
Let us start learning about the experimental techniques in chemistry.
Analytical chemistry
The branch of chemistry which deals with the chemical characterization of a substance is called analytical chemistry.
Definition of analytical chemistry
Complete chemical characterization includes two types of analysis.
(i) Qualitative analysis
(ii) Quantitative analysis
(l) Qualitative analysis
It is concerned with the determination of the nature of the substance. In this type of analysis, we are concerned with the determination of which elements are present in a chemical compound.
(ii) Quantitative analysis
In this type of analysis we are concerned with determination of amount of different element present in a chemical compound.
Steps in quantitative analysis
It involves four major steps,
- Obtaining a sample for analysis
- Separation of the desired constituent
- Measurement and calculation of the result
- Drawing conclusion from the analysis
Purification techniques in chemistry
Filtration
It is a process by which insurable particles are separated from the liquid.
Definition of filtration
The insoluble solid particles obtained after filtration are called residue.
During filtration, the liquid that passes through the filter medium is known as filtrate.
The medium used for filtration is called a filter medium.
Points for a good filtration process
- The filter paper must be so large that it is ¼ to ½ full of the total precipitate at the end of filtration.
- The funnel should be so large that its upper rim is approximately 2 cm above the edge of filter paper.
- The stem of the funnel should be several cm long so that it goes down into the beaker.
Precaution for good filtration
To run the filtration smoothly, the stem of funnel is kept full of liquid, till there is liquid present in conical part of the funnel.
The tip of the stem of funnel should touch the side of beaker, so that the filtrate runs down the side of beaker without splashing.
Types of filter media
It can be performed by different types of filter media.
The nature of precipitates nature of solvent and nature of solvent determine which type of filter media should be used.
The most commonly used filter media are…
i) Filter paper
ii) Filter crucible
Folding of filter paper
- The filter paper should be folded twice.
- First, fold the paper in half along the diameter of the paper.
- Second fold the paper in half again, such that the edges of the paper do not quite match
- Open the paper on a slightly larger section such that three-fold thickness is on the one-half side and one fold thickness is on the other half side and apex angle, slightly greater than 60 degree.
- Wet the filter paper with water and insert it into a 60-degree funnel and firmly press down.
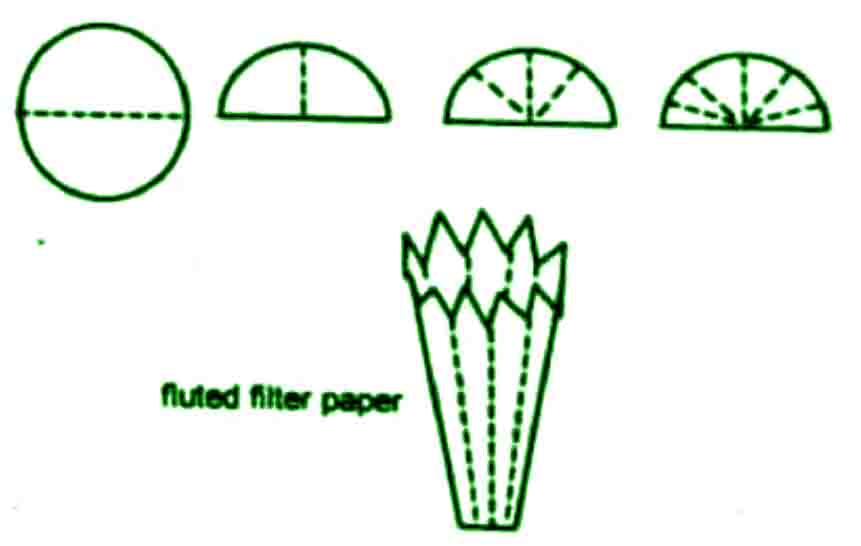
Fluted filter paper
The rate of filtration could be increased by using fluted filter paper
For this purpose ordinary filter paper is folded in such a way that there is a friend like arrangement with alternate elevation and depression
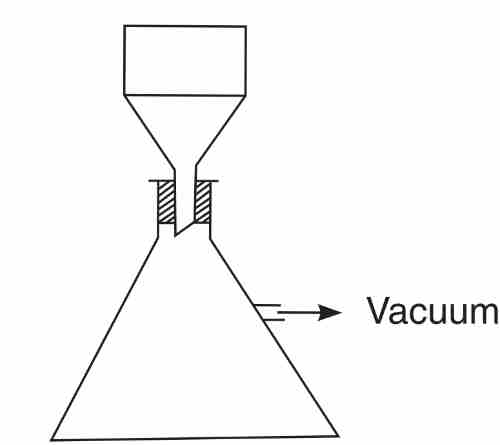
Filter crucible
It is a method to different filter precipitate through suction.
Two types of crucibles are used
Gooch crucible
It is made of porcelain. It has a perforated bottom.
The bottom is covered with filter paper filter paper
The good cross evil is placed in suction filtering apparatus
Advantages of using good crucible
It is useful for filtering precipitation which are to be ignited at higher temperature.
Its perforations are covered with asbestos mat then it may be used to filter solution which filters with paper for example solution containing concentrated HCl, K2CrO3, etc.
Sintered glass crucible
Sintered glass crucible is a glass crucible which have porous glass disc sailed into the bottom.
- It is quite easy to use because no preparation is needed for using it.
- No filter paper is used
- Reactive materials such as HCL, KMnO4 can be easily filtered.
Crystallization
The process of obtaining crystals of a substance by cooling its hot saturated solution is known as crystallization.
Why do we need of crystallization
In most synthetic reactions, chemical compounds are obtained in an impure form. It is purified by the crystallization from a suitable solvent. This crystallization is used to purify the crude product.
The basic principle of crystallization
The solute should be more soluble in the solvent. When the solution is gold the solute should be separated as crystals.
Process of crystallization
There are multiple steps in in process of crystallization.
1: Choice of solvent
The solvent is to zone on hit and trial basis. A number of solvents are tried before reaching the conclusion. Choosing a solvent is very important step in crystallization because whole the process of crystallization depends upon the solvent we use.
Characteristics of good solvent for crystallization
An ideal solvent should processes following characteristics
- It should dissolve a large amount of solvent at its boiling point and only a small amount at room temperature
- It should not react with the solute chemically
- It should not deserve impurities. If impurities are soluble then these should not crystallize along with the desired substance when set to cooling.
- On cooling solution, vel form crystals of parent compound must be obtained.
- It should be inexpensive are cost-effective
- It should be safe to use and should be easily removable.
Commonly used solvents for crystallization
Solvents which are mostly used for crystallization are given below
- Water
- Absolute ethanol
- Diethyl ether
- Rectified spirit
- Carbon tetrachloride
- Acetic acid
- Petroleum ether
Combination of solvents to be used for crystallization
If none of the solvent is found suitable for crystallization, a combination of two or more miscible solvents can be used.
If solvent is inflammable then prefer water bath while heating
2: Preparation of a saturated solution.
- The substance should be dissolved in a minimum amount of the specific solvent
- It is then heated directly or on a water bath with constant stirring.
- More solvent should be added to the boiling solution in order to dissolve the complete solute.
3: Filtration
- The insoluble impurities are removed by filtering hot saturated solution.
- The solution is filtered hot to avoid premature crystallization of the solute on the filter paper or in the stem of the funnel.
- Hot water funnel may be used for this purpose.
4: Cooling
The hot filtered solution is cooled at a moderate rate in order to obtain medium-sized crystals.
Slow cooling gives bigger sized crystals, which usually contain solvent with impurities. Such crystals are difficult to dry.
5: Collecting the crystals
- When crystallization is complete the mixture of crystals and mother liquor is filtered through gooch crucible using a vacuum pump.
- Full suction is applied in order to remove maximum liquor from the crystals.
- If filter cake is hard it is pressed firmly with a cork to remove the remaining mother liquor.
- Crystals are then washed with a small amount of cold solvent several times.
- The mother liquor is often concentrated by evaporation. It is then cooled to obtain a fresh new crop of crystals.
- The efficiency of the crystallization process depends upon the percentage of pure material obtained from crude substances.
6: Drying of crystallized product
There are three methods of drying crystals.
Pressing of crystals using filter paper
By pressing the crystal between folds of filter paper for multiple times.
Disadvantages of pressing crystal between for the printer paper
The crystal are crushed to a fine powder
Fibers of fitter paper contaminate the product
By using oven
The crystals are dried in an oven provided the substance does not melt are decompose on heating at a hundred degrees centigrade.
Vacuum desiccator
It is safe and reliable method of drying the crystals. In this method the crystals are separate over of water class and placed in the vacuum desiccator in the presence of dehydrating agent calcium chloride silica gel are phosphorus pentoxide.
7: Decolorization of undesirable color from crystal
Sometime crystal become coloured due to the presence of some impurities. In this case these crystals are wild with sufficient quantity of animal charcoal. Animal charcoal observe the coloured impaired and pair decolourised crystals are separated.
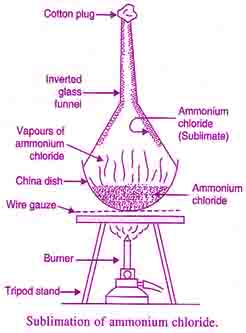
Sublimation
The process of direct conversion of a solid into vapors by heating without passing through the liquid is known as sublimation.
Solid ⇌ Vapour
Solids which goes under sublimation.
There is an important concept of triple point where all three phases of matter exists at the same time.
Iodine, NH4Cl, naphthalene, anthracene, benzoic acid, etc undergoes the process of sublimation.
Process of sublimation
- The impure substance is taken on a watch glass.
- It is covered with an inverted funnel having a cotton plug into its stem.
- The funnel is cooled with wet cotton.
- The substance is heated slowly on a sand bath.
- The pure solid deposits on the inner cold side of the funnel.
Solvent extraction
The process of obtaining a substance from a solution with the help of an immiscible solvent is called solvent extraction.
Definition of Solvent extraction
Principle of solvent extraction
The process of solvent extraction is totally based on Distribution law.
By this process a solute can be separated from a solution by an immiscible solvent. The desired solute is more soluble in solvent than the solution.
Therefore, the solute will move from solution to the added solvent layer and this layer can be separated. The solute can be obtained by evaporating the solvent.
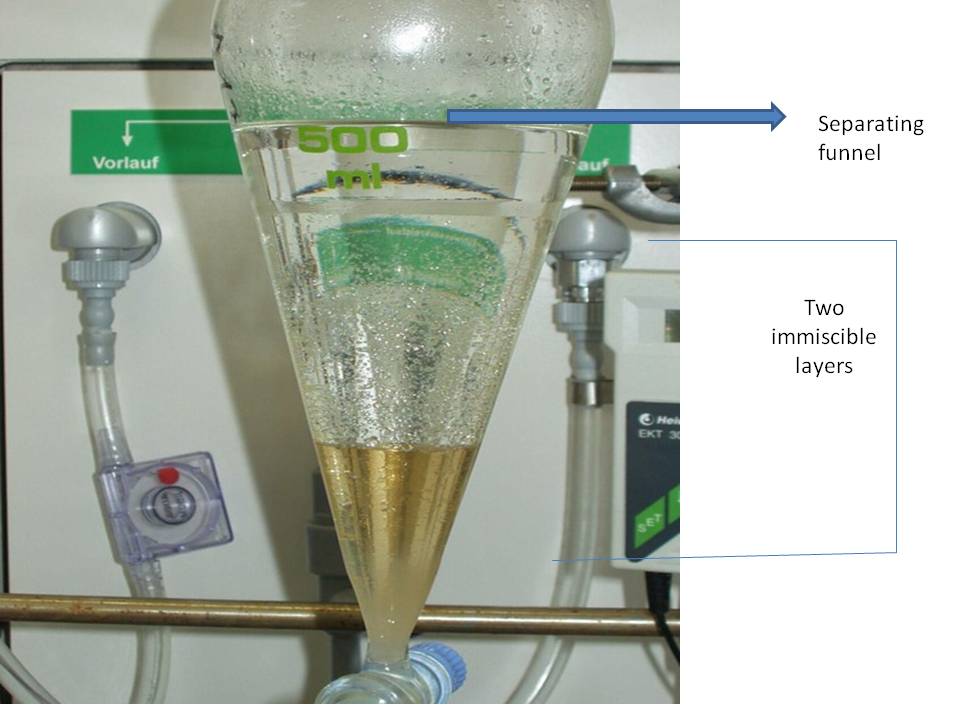
Example of solvent extraction
Ether extraction, a commonly used laboratory solvent extraction.
It is mainly used to separate organic compounds from water.
Process of ether extraction
- The aqueous solution containing an organic compound is shaken up with ether in a separating funnel.
- The organic compound will move from the water layer to the ether layer. The inorganic impurities remain in the aqueous layer.
- The ether layer is separated from the separating funnel.
- After this, the ether layer is evaporated to get the pure organic compound
- Multiple repetitions of this process using smaller portions of solvent give better results than using single extraction with a larger volume of solvent.
- This technique is particularly useful to obtain a product that is volatile or thermally unstable. Such products are difficult to obtain by other techniques.
Chromatography
The term chromatography was derived from the Greek word “Khromatos” which means colour writing.
It is an analytical technique used for the separation of a mixture, due to the difference in the distribution of substances, between a stationary phase and a mobile phase.
Definition of chromatography
Stationary phase
It is known as the phase on which mobile phase flows during the process of chromatography. It may be a solid or liquid that is supported on an inert solid. For example, Silica gel, water adsorbed in paper etc.
Mobile phase
It is known as the phase that flows over the stationary phase. It may be a liquid or gas only. For example, organic solvents Ethanol, Acetone, Ethyl acetate, hexane etc.
The selection of mobile phase depends upon the types of compounds to be isolated. If the compound to be separated are polar than polar solvents such as methanol are used.
If the compounds to be separated are non-polar in nature than non polar solvents such as Hexane is used.
Principle of chromatography
It is based upon distribution law
The mixture is allowed to come in contact with two phases, a stationary phase, and a mobile phase. Different components have different affinities for the stationary phase and mobile phase due to which they are separated.
The distribution of the component between two phases is controlled by distribution coefficient K given as
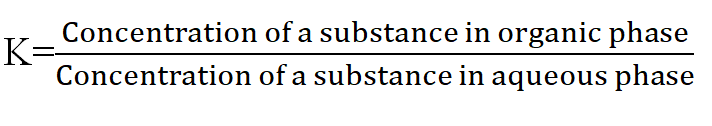
By distribution coefficient we can predict which phase the compounds will stuck on.
As the compounds with smaller K values remain with the stationary phase.
While compounds with larger K value go with the mobile phase.
Classes of chromatography
Chromatography is divided into many classes. Two important classes of chromatography are
1) adsorption chromatography
2) Partition chromatography
Adsorption chromatography
In this chromatography, stationary phase is a solid one.
When the mobile phase flows over the stationary phase, the substance leaves the mobile phase and adsorbed on the stationary phase.
Partition chromatography
In partition chromatography, the stationary phase is a liquid phase supported on the inert solid.
Inert solid means that cannot react with the mobile phase.
In this a substance distributes itself between the mobile phase and the stationary phase. For each class of chromatography many methods can be used. For example, partition chromatography can be performed by the paper chromatography.
Difference between Partition chromatography and adsorption chromatography
| Partition chromatography | Adsorption chromatography |
| It has stationary phase in liquid form | It has solid stationary phase |
| Solute distributes itself between two phases | Solute particles are adsorbed on the solid stationary phase during separation |
| For example: Paper chromatography | For example: Thin layer chromatography |
Paper chromatography
It is a technique of partition chromatography where:
the stationary phase is a liquid adsorbed on a paper.
The mobile phase is liquid passing over the adsorbed liquid in paper, It is usually an organic liquid such as Ethanol, acetone etc.
Paper chromatography can be done in mamy ways
- Ascending
- Descending
- Radial or circular
Ascending paper chromatography is most commonly used technique.
Ascending paper chromatography
In this technique, solvent is placed at the bottom of a vessel. A paper is suspended in it. The solvent moves upward by capillary action.
Process of Ascending chromatography
- A solvent or mixture of solvents is filled in a chromatographic tank.
- The tank is covered with a lid so that its inner space is saturated with solvent vapors and becomes homogenous.
- About 20 cm strip of Whatman’s Chromamtographic paper No.1 is taken.
- A line is drawn with a thin pencil about 1.5 cm above from one end of the paper. This is called the baseline.
- To identify components spots of known compounds may also be placed alongside.
- The spots are dried.
- The paper is suspended in the chromatographic tank in such a way that the baseline must be above the level of solvent and the paper is dipped to a depth of 5-6 mm. This arrangement is left for sufficient time.
- When the solvent front has moved to 3/4th of the length of the paper, paper is removed from the tank.
- The solvent front is marked with a pencil and the paper is dried.
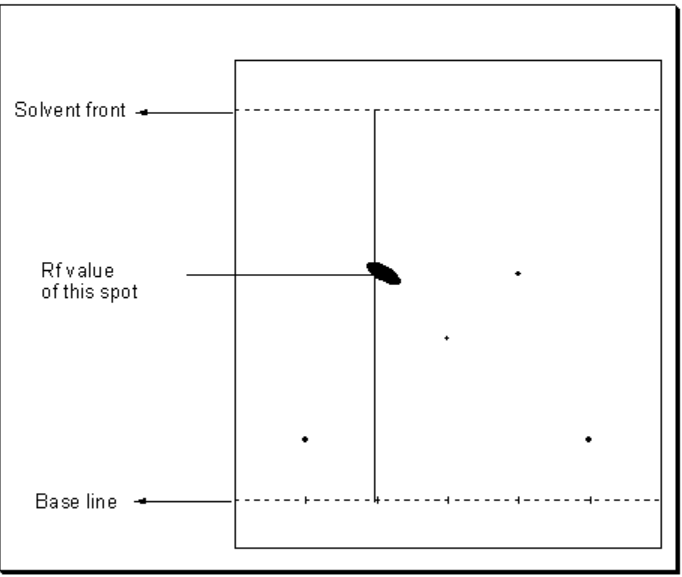
Chromatogram and its development
The pattern of spots on the dried paper is called a chromatogram.
Development of chromatogram
The spots of separated substances can be seen on paper if the substances are coloured.
Otherwise, physical or chemical methods are applied on paper to indentify spots of substances.
These physical methods include the use of different reagents knows as indicators. The indicators are iodine lamp, iodine spray, phenaphthline, etc.
Retardation factor
Each component of a mixture has specific value of retardation factor or retention factor (Rf values).
Rf value is defined as the ratio of the distance travelled by a component from base line to the distance travelled by the solvent from the base line.
Mathematically it can be represented as
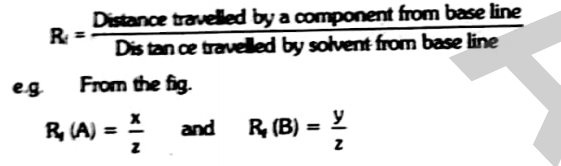
Rf value has no unit as it is the ratio between two similar quantities.
Applications of chromatography
- It is used for the separation of substances from a mixture.
- It is used as a purification technique for compounds.
- It is used for qualitative and quantitative analysis.
- It is used to determine the purity of a substance.