LEARNING OBJECTIVES
In this article, the author has explained what is vapor pressure, dynamic equilibrium between liquid and vapors, factors affecting the vapor pressure with some important examples for better understanding.
Vapour pressure
Table of Contents
The pressure exerted by the vapors in equilibrium with the liquid at a given temperature is called vapor pressure.
Liquid molecules after evaporation are involved in random motion. Due to this motion, they exchange energy among themselves. While moving randomly these exert pressure on the walls of the container in which these are enclosed. This pressure is said to be the vapor pressure.
Watch the video lecture about vapor pressure for a better understanding of this topic.
Let us consider a container enclosed liquid at a certain temperature. High energy molecules in containers leave the surface of the enclosed liquid and gather above the surface in the empty space in the form of vapors.
These molecules collide with the walls of the container as well as with the surface of the liquid. In this way, they lose some of their kinetic energy and there is a chance that these molecules are recaptured by the liquid surface. This process is known as condensation.
Both the process of condensation and evaporation continue till the rates of both the processes become equal. This state is called dynamic equilibrium.
The pressure exerted by the vapors at this state on the liquid surface at a particular temperature is called vapor pressure.
The following diagram gives a clear idea about this dynamic equilibrium between evaporation and condensation.
Dynamic equillibirum
The state at which the rate of evaporation becomes equal to the rate of condensation is called the state of dynamic equilibrium.

At this stage, the number of molecules escaping from the surface of the liquid is equal to the number of molecules recapturing by the surface of the liquid.
Vapour pressure does not depend upon the amount or volume of liquid and surface area.
Factors affecting the vapor pressure
There are certain factors that affect the vapor pressure.
1: Nature of liquid
The Vapour pressure of a liquid depends upon the nature of the liquid. A liquid having low boiling points will show high vapor pressure white liquid with high boiling points will have a w vapor pressure.
Example
The boiling point of ether is lower than ethyl alcohol. Therefore its vapor pressure will be higher than ethyl alcohol at a given temperature.
2: Strength of intermolecular forces
Liquids having stronger intermolecular forces have low vapor pressure while liquids with weaker intermolecular forces have high vapor pressures. Hence there is a very close relationship between the strength of intermolecular attractive forces and vapors pressure.
Due to weaker intermolecular forces, at 20 oC vapor pressure of isopentane is more (580 torr) than glycerol ( 0.00016 torr).
Example
Water has stronger intermolecular attractive forces than carbon tetrachloride. Further water molecule has hydrogen bonding as well. Therefore, water has lower vapor pressure and a higher boiling point than carbon tetrachloride.
3: Size of the molecules
Liquids having greater molecular size have lower vapor pressure and liquids having small molecular size have higher vapor pressure. For example, methane is evaporated much faster than butane because methane molecule size is smaller than butane.
4: Temperature
It is the most important factor that controls vapor pressure. At higher temperatures, the kinetic energy of the molecules is increased. Molecules of higher kinetic energy escape from the liquid more quickly. Thus it causes an increase in vapor pressure.
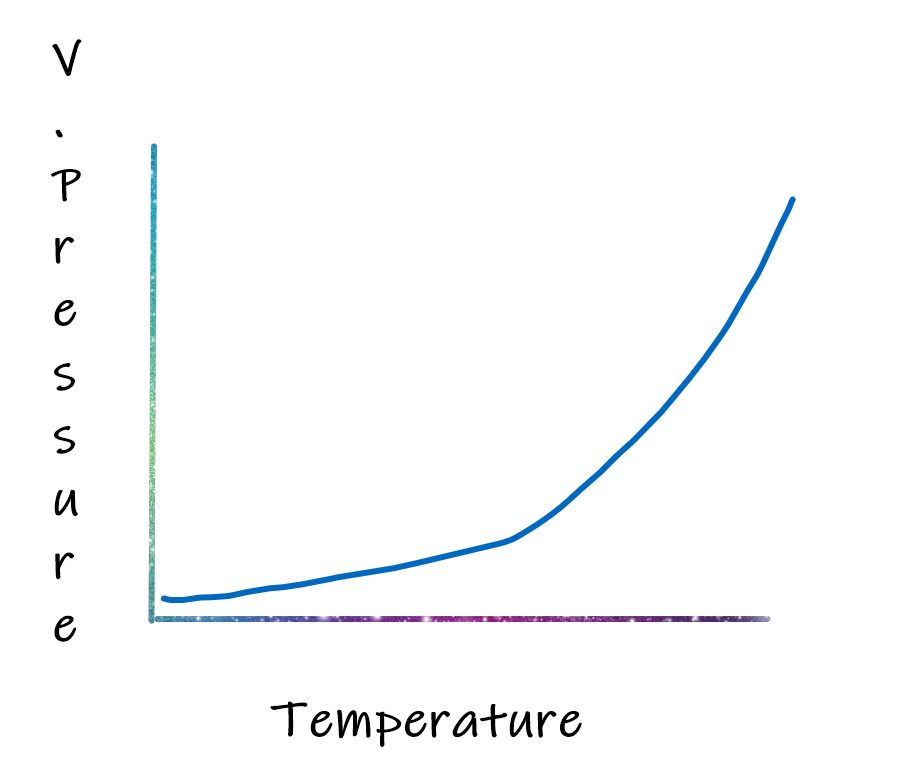
From the table given below, it can be noted that how elevated temperature increases the vapor pressure of water. This table for water indicates that the increase of vapor pressure goes on increasing for the same difference in temperature.
The vapor pressure at 0oC, 4.579 torr, and at 10 oC is 9.202 torr. The difference is 4.6 torr for a 10 oC rise in temperature. When the temperature changes from 90 oC to 100 oC the difference is from 527.8 torr to 760 torr. The rate of change of vapor pressure with temperature is best explained by the Clausius Claperyon equation.
Table
| Temperature oC | Vapour pressure (torr) | Temperature (oC) | Vapor pressure (torr) |
| 0 | 4.579 | 50 | 92.51 |
| 5 | 6.543 | 55 | 118.0 |
| 10 | 9.209 | 60 | 149.4 |
| 15 | 12.79 | 65 | 187.5 |
| 20 | 17.54 | 70 | 233.7 |
| 25 | 23.76 | 75 | 289.1 |
| 30 | 31.82 | 80 | 355.1 |
| 35 | 42.18 | 85 | 433.6 |
| 37 | 47.07 | 90 | 527.8 |
| 40 | 55.32 | 95 | 633.9 |
| 45 | 71.88 | 100 | 760.0 |
Vapour pressure of some liquids at 20 oC
| Name of compound | Formula | Vapor pressure at 20 oC [mm Hg (torr)] |
| Isopentane | CH3-CH2-CH(CH3)-CH3 | 580 |
| Chloroform | CHCl3 | 170 |
| Carbon tetrachloride | CCl4 | 87 |
| Water | H2O | 18 |
| Mercury | Hg | 0.012 |
| Glycerol | C3H8O3 | 0.00016 |
This table showing the vapor pressure of some liquids can help us to compare the vapor pressures of various liquids at 20 oC. Isopentane is a hydrocarbon of non-polar nature. It has the highest vapor pressure. Glycerol has the lowest vapor pressure due to excessive hydrogen bonding.
Vapor pressure related questions
1: Why the vapor pressure of water, ethyl alcohol and diethyl ether are different from each other at 0oC ?
Water, ethyl alcohol, and diethyl ether have different extents of hydrogen bonding in their molecules. Water has maximum hydrogen bonding, ethyl alcohol has less hydrogen bonding while diethyl ether has no hydrogen bonding. For this reason, the vapor pressure of water at 0oC is 4.529 torr that of alcohol is about 15 torr and is 200 torr for diethyl ether at o oC.
2: How dynamic equilibrium is established during evaporation of a liquid in a closed vessel at constant temperature?
We know that the liquid molecules escape from the surface of the liquid and change into vapors. These vapors are recaptured by the liquid and the process is called condensation. At constant temperature the rate of both the processes becomes equal and this is the state of dynamic equilibrium.
Suggested readings
https://byjus.com/chemistry/liquid-state-vapour-pressure/