Written by Adeel Abbas
Benzene is an aromatic compound that is widely used in the chemical industry. It is a cyclic hydrocarbon with a unique stability and reactivity towards difference reagents.
Discovery of benzene
Table of Contents
The discovery of benzene is generally attributed to the German chemist Friedrich August Kekulé. In the 1850s and 1860s, Kekulé proposed that the carbon atoms in benzene were arranged in a ring, with alternating double bonds. He also proposed that these double bonds were delocalized, meaning that the electrons in the double bonds were spread out over multiple atoms.
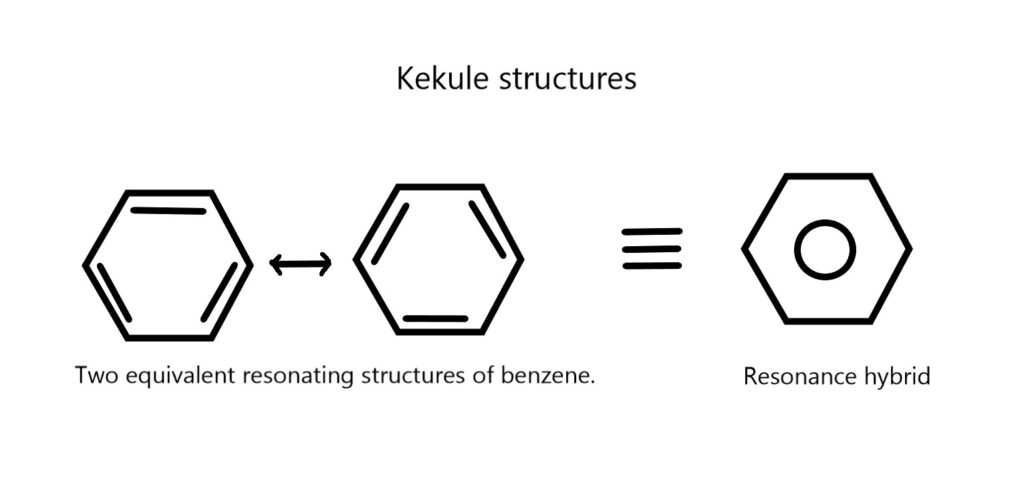
Kekule structure of benzene
Kekulé’s proposed structure for benzene was groundbreaking at the time, as it helped to explain many of the properties of the compound, such as its stability and reactivity. His work on benzene was a major step forward in the field of organic chemistry and helped to establish the field of structural chemistry.
Kekulé’s structural model for benzene was further confirmed by the work of other chemists, such as Joseph Loschmidt, who proposed a similar structure for benzene, and by later experimental evidence, such as the results of X-ray diffraction studies.
Methods for Preparation of benzene
Benzene can be prepared through four methods that are common in the industry.
| Method | Process |
| 1. Cyclic polymerization of Alkynes | Ethyne passes through a tube of red-hot iron at 873K |
| 2. Decarboxylation of Aromatic Acids | Sodium salt of Benzoic acid is heated with Soda Lime |
| 3. Reduction of Phenols | Vapors of Phenol pass over heated dust of Zinc |
| 4. Hydrolysis of Sulphonic Acids | Sulphonic acid is exposed to heated steam |
Stability of Benzene ring
One of the unique characteristics of benzene is its stability. The electrons in the aromatic ring are delocalized, meaning they are spread out over multiple atoms rather than being localized on a single atom. This delocalization results in a stabilization of the ring, making it less reactive than alkenes.
Physical properties of benzene
| Property | Value |
| Molecular Formula | C6H6 |
| Molecular Weight | 78.11 g/mol |
| Density | 0.87 g/cm3 |
| Melting Point | 5.5 °C |
| Boiling Point | 80.1 °C |
| Solubility in Water | Insoluble |
| Refractive Index | 1.501 |
| Vapor Pressure | 43 mmHg at 20 °C |
| Flash point | -11 °C |
| Autoignition temperature | 446 °C |
Chemical properties of benzene
Benzene possess unique chemical properties. There are certain factors that imparts the reactivity into benzene ring. Let us discuss these in detail.
Aromaticity: Benzene is a highly aromatic compound and exhibits resonance stabilization. This means that the electrons in the ring are distributed evenly around the ring and are delocalized, making it more stable and less reactive than other molecules with similar structures.
Reactivity: Despite its aromatic nature, benzene is relatively unreactive towards electrophilic reagents. This is due to the electron-withdrawing effect of the ring’s double bonds, which make the ring less electrophilic. However, it can undergo nucleophilic substitution reactions and electrophilic substitution reactions with a certain reagents such as bromine in the presence of a Lewis acid catalyst.
Oxidation: Benzene is resistant to oxidation and does not easily react with oxygen.
Pyrophoric: Benzene is pyrophoric, which means that it may spontaneously ignite in air.
Toxicity: Benzene is toxic and exposure to high concentrations can have negative effects on human health, including anemia, leukemia and other blood disorders.
Benzene undergoes following types of reactions.
Electrophilic Substitution Reactions
One of the most common reactions of benzene is electrophilic substitution. In this type of reaction, an electrophile (an atom or molecule with a positive or partial positive charge) replace a functional group in the aromatic ring of benzene. This results in the formation of a new compound, with the electrophile replacing one of the hydrogens on the ring.
Nucleophilic Substitution Reactions
Benzene can also undergo nucleophilic substitution reactions. In this type of reaction, a nucleophile (an atom or molecule with a negative or partial negative charge) attacks the electrophilic center of an benzene ring. In the case of benzene, the nucleophile attacks a carbocation that is formed when an electrophile attacks the aromatic ring.
Examples of nucleophilic substitution reactions of benzene
- Reduction of nitrobenzene to aniline: Nitrobenzene is reduced by a nucleophile, such as hydrazine, to form aniline.
- Reduction of diazonium compounds to phenols: Diazonium compounds, such as benzenediazonium chloride, are reduced by a nucleophile, such as hydroxide ion, to form phenols.
- Hydrolysis of alkylbenzenes: Alkylbenzenes, such as toluene, undergo hydrolysis in the presence of a nucleophile, such as water, to form benzoic acid and the corresponding alcohol.
Addition Reactions
Benzene can also undergo addition reactions. In this type of reaction, a compound is added across the double bond of the ring. This can happen via electrophilic or nucleophilic addition.
One example of electrophilic addition reaction is hydrogenation of benzene to cyclohexane. In the presence of a catalyst such as nickel, hydrogen adds across the double bond of the ring and forming a saturated cyclic hydrocarbon, cyclohexane
Another example of addition reaction is electrophilic addition of chlorine to form chlorobenzene. In the presence of Lewis acid catalyst, chlorine adds to the double bond of the ring forming chlorobenzene.
Benzene is a unique and widely used compound in the chemical industry. Its stability and reactivity make it a versatile starting material for a wide range of reactions. Understanding the different types of reactions that benzene can undergo, such as electrophilic substitution, nucleophilic substitution, and addition reactions, is crucial for understanding its properties and applications.
Summary of Benzene reactions
| Reaction Type | Example | Mechanism | Product |
| Electrophilic Substitution | Halogenation | Electrophile (X2 or XBr) attacks the ring in the presence of Lewis acid catalyst | Ar-X (Ar is the substituent) |
| Nitration | Nitronium ion (NO2+) attacks the ring in the presence of sulfuric acid | Ar-NO2 | |
| Nucleophilic Substitution | Reduction of Nitrobenzene | Nucleophile(NH2NH2) attacks the electrophilic center of the nitro group | Ar-NH2 |
| Addition | Hydrogenation | Hydrogen added across the double bond in the presence of a catalyst | Cyclohexane |
| Chlorination | Chlorine added across the double bond in the presence of a Lewis acid catalyst | Chlorobenzene |
Note:
Ar indicates benzene ring attached with an other electrophile.
The specific conditions and catalysts for each reaction can vary depending on the exact process being used.
Uses of Benzene
| Use | Applications |
| Solvent | Industrial and commercial, used as absorbing and binding agent |
| Rubber Production | Tires, rubber products |
| Paints and Printing | Cleaning equipment, inks, dyes, lacquers, stains, sealers |
| Fuel | gasoline, lubricants, asphalt |
| Chemical and Plastic Production | Resins, adhesives, synthetic fibers, detergents, dyes, insecticides, pesticides and herbicides |
| Auto Repairs | Cleaning and maintenance of machinery |
| Pharmaceuticals | Production of drugs and medications |
| Agriculture | Pesticides and fertilizers |
| Electronics | Production of semiconductors |
| Food and Beverage Industry | Additives and preservatives |
| Adhesives | Glues and sealant production |
| Synthetic fragrances | perfumes and other scented products |
| Explosives and Fireworks | TNT, dynamite, and other explosive materials |